Swiss Vascular Biobank (SVB)
Collecting biological tissue samples in a biobank grants a unique opportunity to validate diagnostic and therapeutic strategies for translational and clinical research.
In order to explore extensively pathophysiological changes in the diseased vessels, a vast number of tissue samples is necessary due to the heterogeneous properties of vascular disorders. Thus, one of the most important goals of our research group, along with the Research Biobanking Service Center of USZ, is to establish a high-quality Swiss Vascular Biobank that would match the state-of-the-art requirements at an international level.
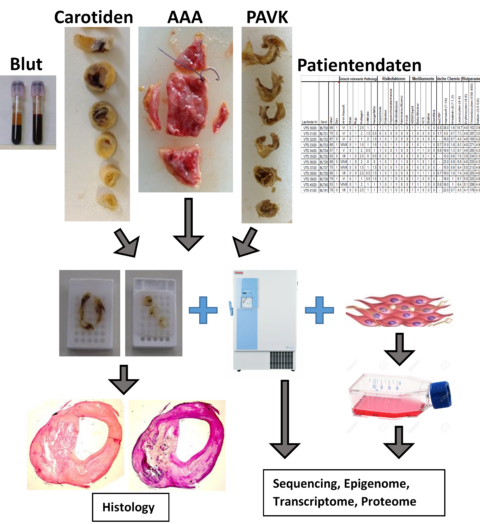
From all patients where possible, who undergo surgical intervention in our Department of Vascular Surgery, agree signing the General Consent and fulfil our inclusion criteria, excessive vascular tissue is removed, segmented accordingly and stored appropriately in our Vascular Biobank. Depending on size and quality of the tissue, consecutive segments are prepared and segmented. One part is directly frozen in liquid nitrogen, stored at -80°C and used for molecular-biological analyses (transcriptomics, proteomics, RNA-sequencing, single-cell RNA-sequencing, qRT-PCR, western blotting, arrays). Onother part is used to isolate primary patient-derived SMCs and ECs, and one part is fixed in formalin and embedded in paraffin (FFPE) and used for sample characterisation and immunohistochemistry. In addition, blood of the patients is collected as well, together with patient’s demographic data and medical history.
Following vascular tissue biomaterial is collected:
- Thoracic and abdominal aortic aneurysm (TAA, AAA)
- Aneurysm of iliac or popliteal arteries (IA, PA)
- Aortic Arch (AB = Aortenbogen)
- Carotid plaques (Carotid Artery Stenosis, CAS)
- Peripheral artery Disease (PAK)
- Non-aneurysmal aortic wall following kidney transplantation (Department of Visceral- and Transplantation Surgery)
- Pelisek J, et al. Biobanking: objectives, requirements and future challenges – experiences from the Munich Vascular Biobank. J Clin Med. 2019;8(2). pii: E251.