Overview
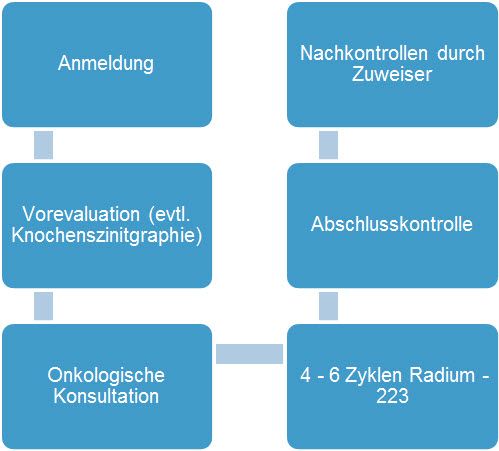
Radium-223 was approved in Switzerland in 2014 with the help of the University Hospital Zurich. Ra-223 is an analog of calcium and is deposited in active bone metastases. Due to the high and pinpoint irradiation of radium-223, all bone metastases in the body are treated with a high level of local irradiation. Since radium-223 is a so-called alpha emitter, only about 10 cell diameters are treated locally. The indication is the treatment of patients with castration-resistant prostate cancer (CRPC) and symptomatic bone metastases without known visceral metastases if chemotherapy is not indicated or in case of progression after docetaxel.