Research Group: Protein Misfolding (Group leader: Dr. med. Marco Luciani)
In order to elicit physiological functions, proteins must follow an exact sequence of folding regardless of localization, turnover and molecular size. In case this is not respected, proteins can acquire erroneous spatial conformations by being folded in wrong ways and potentially eliciting cell stress.
Luckily, cells are equipped with molecular machineries devoted in tagging, unfolding, refolding or degrade disarranged proteins (monomers). If these systems are overwhelmed, monomers will accumulate leading to the formation of oligomers and ultimately to amyloid fibers thus impairing cell, tissue and organ functions (Fig.1).
This axiom called protein folding, in case of derangement, is the biological foundation of aging systems characterizing various chronic neurodegenerative diseases as Alzheimer’s dementia and it has been recently recognized as a driving mechanism of damage in a number of cardiovascular diseases such cardiac amyloidosis and several others.
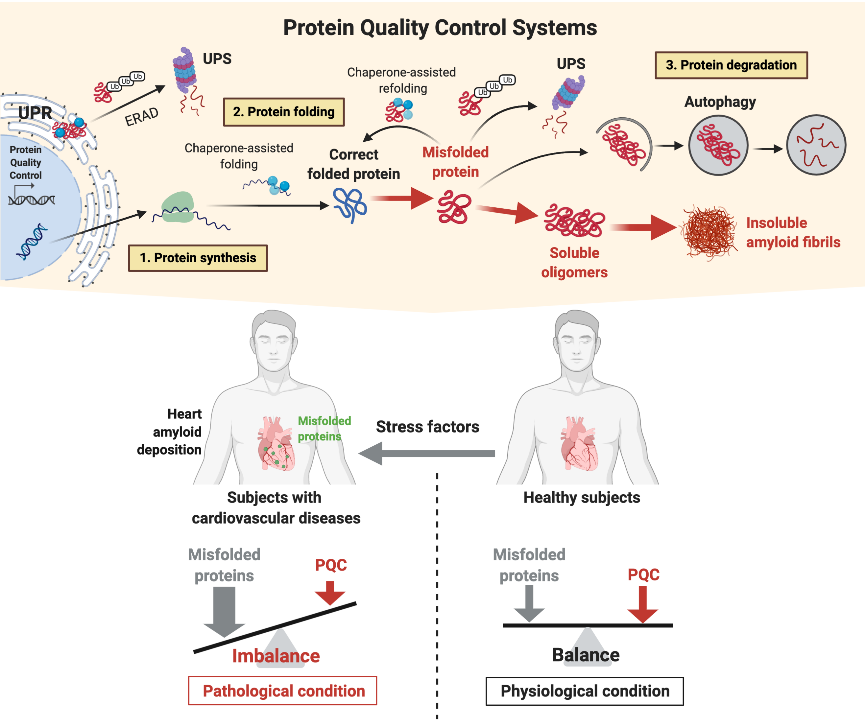
Fig. 1. Protein quality control system in health and disease
Ongoing research lines
- Natriuretic peptides biology
- Heart-and-Brain crosstalk
- Protein misfolding and identification of therapeutical targets
- Key triggers leading to cardiac amyloidosis development and progression
Research Team
- Thamonwan Diteepeng, PhD student
Funding
- University of Zurich
- Schweizerische Herzstiftung/Swiss Heart Foundation
- Olga Mayenfisch Stiftung
- Diteepeng T, del Monte F, Luciani M. The long and winding road to target protein misfolding in cardiovascular diseases. Eur J Clin Investig. 2021;51:e13504
- Luciani M, Troncone L, del Monte F. Current and future circulating biomarkers for cardiac amyloidosis. Acta Pharmacologica Sinica. 2018;39:1133-1141
- Troncone L*, Luciani M*, Coggins M, Wilker EH, Ho CY, Codispoti KE, Frosch MP, Kayed R, del Monte F. Aβ Amyloid Pathology Affects the Hearts of Patients With Alzheimer’s Disease: Mind the Heart. J Am Coll Cardiol. 2016;68:2395-2407.