Research Group: Cardiovascular Aging (Group Leader: Dr. Soheil Saeedi)
Senescence of endothelial cells and the neighboring tissue, perivascular adipose tissue (PVAT), is a bona fide risk for aging-associated cardiovascular diseases. Redox imbalance is known as a key driver of cardiovascular aging, while much remains unknown about the intrinsic origins and environmental factors which may trigger detrimental alterations eliciting senescence in vascular and perivascular adipose cells.
As we age, our cardiovascular system undergoes significant changes that can contribute to the development of cardiovascular diseases such as atherosclerosis and heart failure. These changes include senescence in diverse cell types, namely endothelial cells (EC) and perivascular adipocytes (PVA), in cardiovascular system. The senescent cells become pathogenic and taint neighboring healthy cells by extensive alterations in redox signaling, energy homeostasis, epigenetic signals, and metabolic function. Recent research reveals that ECs and PVAT interact through their senescence-messaging secretome (SMS). So, what promotes senescence in one can stimulate cellular senescence and dysfunction in the other one. Multiple initiating stimulators such as gut microbiota-derived metabolites have been identified to drive oxidative stress, a major hallmark of cellular senescence, and regulate oxidative-regulated host genes involved in the biology and function of ECs and PVAT. We recently discovered that aging-related microbiota alterations negatively impact redox and energy homeostasis and epigenetic signals, and thereby cause senescence in these cells. Whereas the majority of investigations have been on therapies related to cardiovascular disease in elderlies, during the recent decade, an emerging shift towards deciphering mechanisms driving senescence in cardiovascular system and characterizing different senotherapies occurred.
The Saeedi Lab investigates the redox signaling and microbial metabolites orchestrating senescence in ECs and PVAT and the subsequent cardiovascular aging by employing the state-of-the-art multi-omics, chemogenetics, molecular biology, wild-type and GEM mice, and unique aging human cohorts (Fig. 1). The lab also provides new insights into potential senescence-escape therapies including (1) metabolite-based and (2) chemogenetic approach that may indeed restore youthful-like (peri)vascular function and extend lifespan (Fig. 2).
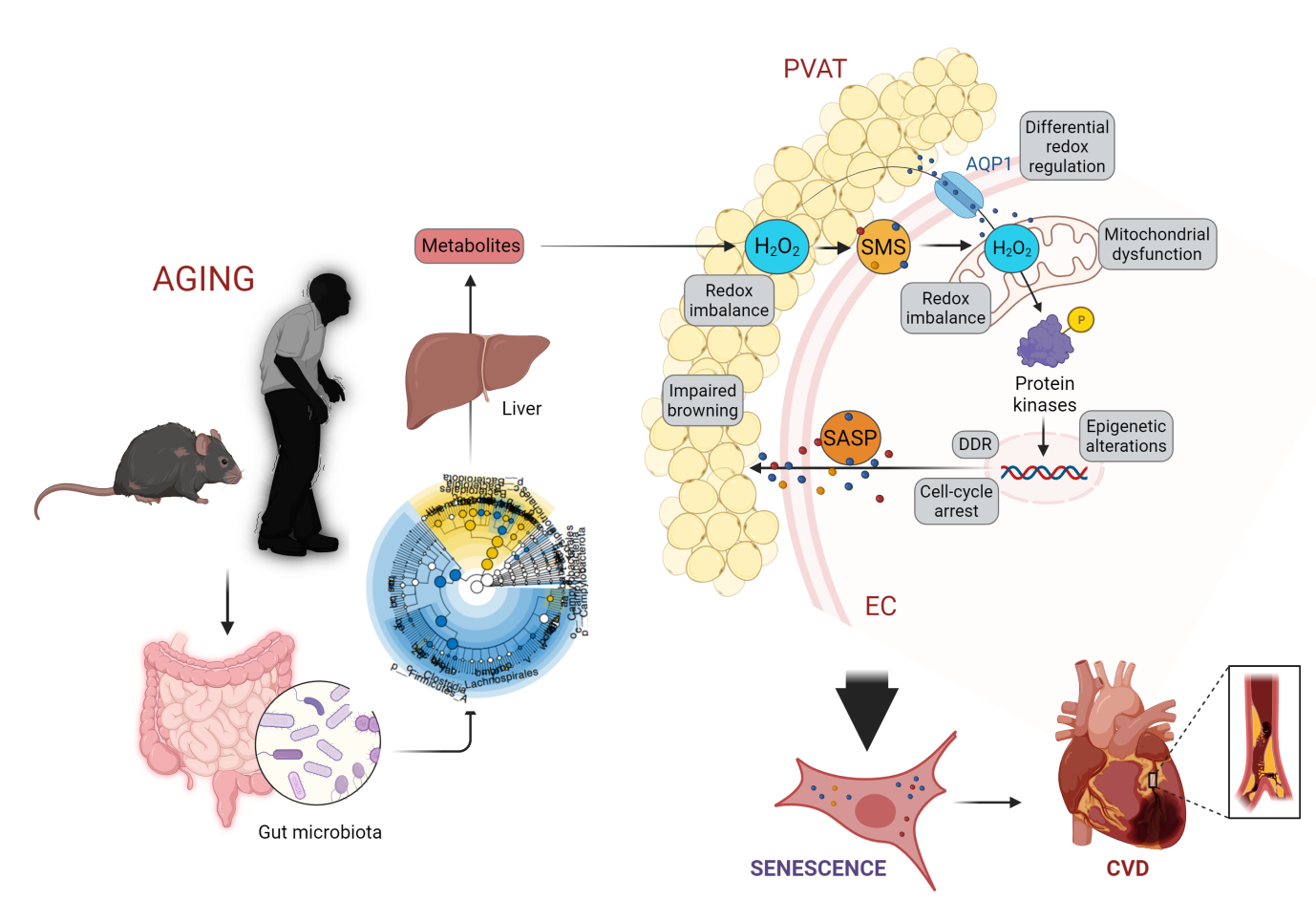
Fig. 1. Gut microbiota-redox machinery orchestrates EC-PVAT senescence and subsequently cardiovascular aging
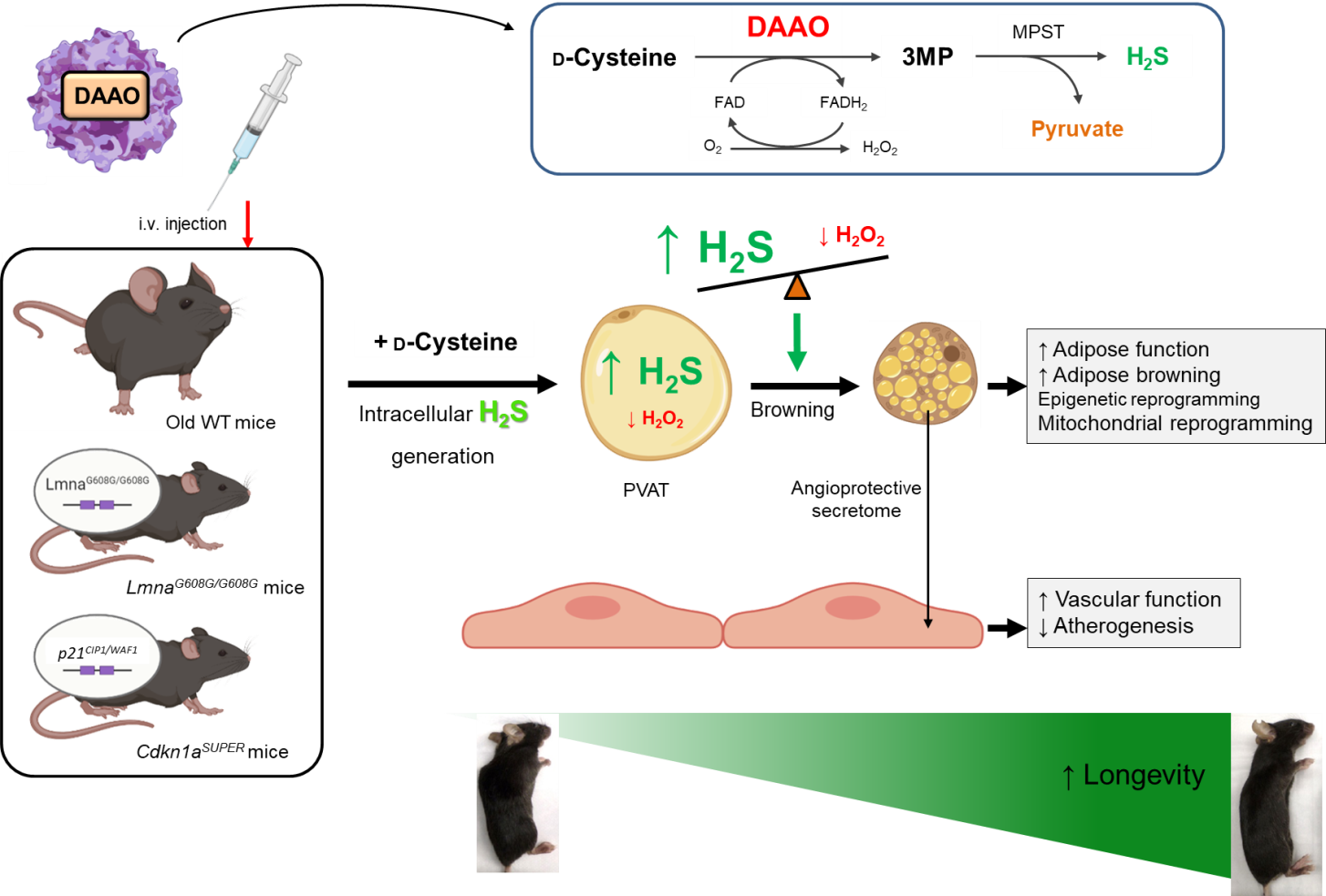
Fig. 2. Chemogenetic approaches to restore redox homeostasis in aging PVAT and rescue EC senescence in animal models of premature aging
Ongoing research lines
- Investigate the mechanisms orchestrating redox homeostasis in endothelial cell and PVAT senescence
- Understand the roles of gut microbiota-derived metabolites in regulating oxidative cellular senescence in (peri)vascular cells
- Identify microbial metabolites as novel prognostic biomarkers and therapeutic targets for cardiovascular diseases in aging
- Develop chemogenetic and senotherapeutic approaches for reprogramming aged (peri)vascular cells
Research Team
Sarah Simone (Practical Student)
Recruitment is ongoing
Funding
Fonds zur Förderung des akademischen Nachwuchses (FAN), PI
Novartis Foundation for Biomedical Research, PI
Gebauer Stiftung, PI
Jubiläumsstiftung von SwissLife, PI
Stiftung Kardio, PI
Awards & Honors
- Paul Dudley White International Scholar Award, American Heart Association (2021 & 2022)
- Young Investigator Fellowship, European Atherosclerosis Society (2021-2023)
- DOCR Award, 21st Day of Clinical Research (DOCR), University of Zurich (2022)
- The Peter Hans Hofschneider Professorship Finalist (2022)
- The Helmut Horten Professorship Finalist (2022
- AGLA Walter Riesen Award, Swiss Atherosclerosis Association (2021)
- ATVB Grant for Early Career Investigators, American Heart Association (2021)
- HMPA Prize, Harvard Medical School (2018)
- Postdoctoral Research Fellowship, Harvard Medical School (2017-2019)
- Saeedi Saravi SS, Lee P, Allemann M, Beer JH. Targeting redox system for cardiovascular regeneration. Aging Cell. 2023; Accepted
- Saeedi Saravi SS, Bonetti NR, Pugin B, Constancias F, Pasterk L, Gobbato S, Akhmedov A, Liberale L, Lüscher TF, Camici GG, Beer JH. Dietary omega-3 fatty acid suppresses age-associated thrombotic potential via gut microbiota modulation. iScience. 2021; 24: 102897
- Saeedi Saravi SS, Eroglu E, Waldeck-Weiermair M, Sorrentino A, Steinhorn B, Belousov V, Michel T. Differential endothelial signaling responses elicited by chemogenetic H2O2 Redox Biol. 2020; 36:101605
- Eroglu E*, Saeedi Saravi SS*, Sorrentino A, Steinhorn B, Michel T. Discordance between eNOS phosphorylation and activation revealed by multispectral imaging and chemogenetic methods. Proc. Natl. Acad. Sci. USA. 2019; 116: 20210-20217
- Sorrentino A, Steinhorn B, Troncone L, Saeedi Saravi SS, Badole S, Eroglu E, Kijewski MF, Divakaran S, Di Carli M, Michel T. Reversal of heart failure in a chemogenetic model of persistent cardiac redox stress. Am. J. Physiol. Heart Circ. Physiol. 2019; 317: H617-H626.
- Spyropoulos F, Sorrentino A, van der Reest J, Yang P, Waldeck-Weiermair M, Steinhorn B, Eroglu E, Saeedi Saravi SS, Yu P, Haigis M, Christou H, Michel T. Metabolomic and Transcriptomic Signatures of Chemogenetic Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2022; 322: H451-H456.
*contributed equally