Preanalytics of the University Center for Laboratory Medicine and Pathology
A topic that unfortunately often receives too little attention is preanalytics, i.e. all the partial steps from obtaining the sample to its measurement. Errors during this time are the most common cause of clinically implausible results.
Since most of the sub-steps are beyond the control of the laboratory, it must be pointed out that the analytical results and findings of the IKC, HAD and AKI are only valid under the restriction that the specifications for preanalytics have been implemented correctly.
Blood
The quality of laboratory tests is strongly influenced by parameters and interfering factors, which can be controlled or excluded by correct procedures during patient preparation, blood collection, sample storage and transport.
- Physical measures: Heavy physical stress and some therapeutic and diagnostic measures, such as intramuscular injections and prostaticalpation lead to the increase of various enzymes and substrates. Specifying the sampling time is particularly important for parameters that are subject to circadian rhythms (for example, iron and cortisol).
- Food intake: Very many parameters are changed by food intake. Reference ranges are for blood samples obtained in the fasting state (at least 8 hours postprandial). Therefore, specimen collection is ideally performed with the patient fasting. If this is not possible, the laboratory results must be interpreted with appropriate reservation. There are also special dietary rules for some parameters.
- Drugs: (Including plasma expanders) They show interference with their metabolites by intrinsic color (rifampicin, anthraquinones), by fluorescence (tetracyclines), by reducing properties (ascorbic acid, dopa), by chelation (phenothiazines), or by influence on plasma protein binding (hormonal contraceptives). In general, it should be noted that drugs can influence the results of laboratory analyses not only by methodological interferences, but that more often unknown or unknown substances can also influence the results. unexpected pharmacological effects lead to changes in vivo.
- Venous congestion: it must be applied only for a short time. One minute should not be exceeded, otherwise cells and macromolecular substances such as proteins (e.g. enzymes) and the molecules bound to them (e.g. lipids, bilirubin, hormones, drugs, iron, calcium, magnesium) will be concentrated. An upright posture has the same effect, which is why blood collection in a sitting or lying position is recommended.
- Repetitive fist closure: “pumping” during blood collection results in an increase in potassium and magnesium.
- Insufficient filling of the tube: This can lead to incorrect results. The tubes must be filled up to the indicated mark (small triangle above the colored bar) or up to above the bar. The reason for this is the prescribed mixing ratio of whole blood and additive. This is particularly important for coagulation tubes, where small deviations already lead to massive analytical errors. Carefully tilt the Vacutainer with additives five times after blood collection so that the additives are mixed with the blood.
- Anticoagulant additives in the collection tubes: They can interfere with some tests. The color coding on our order forms must therefore be strictly observed and the materials should be obtained in the correct order when taking blood samples (see under Sample Vessels). Never pour blood from one type of tube into another!
- Hemolysis: In plasma/serum it leads (by incorrect collection, by insufficient mixing, by incorrect storage, etc.) to increase of potassium and a number of enzymes, e.g. LDH, CK, AST. In addition, the intrinsic staining caused by heme interferes with a number of color reactions (e.g., P-amylase) as well as with single factor determinations (coagulation).
Common causes of hemolysis are:- Blood collection from catheters and too small or too large lumen cannulas
- Congestion too long (max 1 minute: open congestion after puncture for aspiration).
- Aspiration of disinfectant residues into the specimen: The disinfectant must be dried before puncture.
- Icteria: It can interfere with absorbance measurements in the 400-500 nm range and with some immunological methods (FPIA).
Lipemia of plasma/serum leads to an apparent decrease in electrolytes (sodium, potassium, calcium) due to displacement effects. Turbidity can interfere with turbidimetry and absorbance measurements. Highly lipemic samples are ultracentrifuged in the laboratory (except for the determination of cholesterol, triglycerides, ammonia and alcohol). - Storage and transport: In principle, a sample should not be stored, but handed over to the transport service immediately after acceptance. An excessively long interval (longer than one hour) between blood collection and separation of the cellular components will result in an increase in potassium and loss of glucose in the heparin plasma or serum, among other effects. If storage is unavoidable, we recommend room temperature and darkness. The sample must be kept sealed, otherwise evaporation (even in the refrigerator!) will concentrate almost all parameters!
- Trace element tube: It must not be opened under any circumstances after blood collection (risk of contamination). The same line should not be used for blood sampling for drug level determinations as for infusion of the same drug.
Collection of test blood: Blood units will only be issued after two independent blood group determinations. The exception is an acute emergency with an immediate need for transfusion.
Out-of-town blood group determinations are accepted.
- Independent means: two separate blood samples are needed. Order stickers must be directed by the person taking the blood sample, patient is asked again for name, first name and date of birth.
- With her identifier on both the blood tube and the order form, the nurse assumes responsibility for the correct identification of the patient and is therefore legally liable.
- If the blood group is already known, only one blood sample (test blood) needs to be taken to perform the antibody screening test.
- Citrate tubes (light blue) for coagulation analyses (e.g. Quick) must absolutely be filled up to the mark to enable correct results. Foam formation during removal must be avoided. Under no circumstances should the contents be completed with another tube. If the Vacutainer is incompletely filled, it must be pulled away, the tube carefully tilted five times and then put back on and the missing residual volume filled up. If necessary, a sample must also be taken again.
- If the hematocrit is > 0.6 l/l, specially adapted blood collection tubes (citrate) that can be obtained from the coagulation laboratory must be used.
- Specimens must be transported and stored under appropriate conditions (e.g., no storage in refrigerator).
- Samples for PFA closure time and platelet aggregation must not be sent by pneumatic tube, but must be forwarded immediately to the laboratory by transport service.
Urine
- The 1st morning urine collection time is particularly suitable for bacterial tests, test strips, nitrite tests, sediment, protein diagnostics and clinical chemistry tests.
- The 2nd morning urine is suitable for test strips, glucose or proteins, but not for nitrite tests
- Spontaneous urine is only useful in acute situations and is unsuitable for bacterial or microbial testing
This form of urine is particularly suitable for bacterial testing.
The sampling time corresponds to a defined collection period, usually 24 hours. Collected urine is suitable for clinical chemistry studies, but it is unsuitable for bacterial or microscopic studies.
Spontaneous urine samples are more suitable for qualitative statements. For most questions, midstream urine is required. Precise instructions from the patient are necessary here in particular:
- Cleaning hands and genitals
- Collection of the midstream without interruption of micturition

Obtaining midstream urine in men
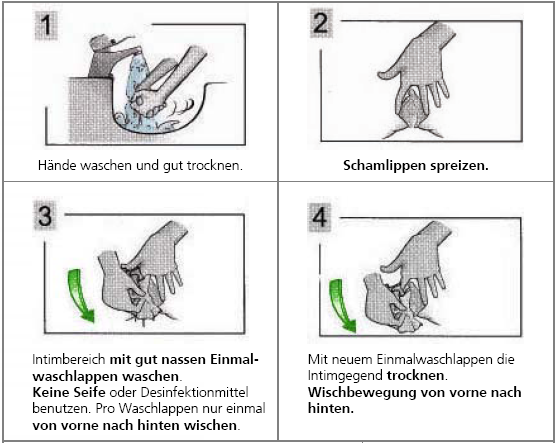
Obtaining midstream urine in women
To ensure the determination of correct and plausible results, it is essential to comply with the relevant collection regulations. In particular, the patient must be instructed precisely:
- The first morning urine should be discarded. The smamle period begins with the second morning urine.
- Pour all micturitions into the collection vessel during the day and the following night.
- The first morning urine of the next day is collected as the last portion.
- Enter collection time and urine volume on the order form.
- Mix the collected urine well and fill approx. 100 ml into urine cups with screw cap.
In some cases it is necessary to collect the urine protected from light and/or cooled (see UZL analysis information system).
For several tests in collected urine, special additives are needed to prevent it from precipitating or being metabolized during collection. The table provides an overview. Exact regulations can be found at the respective parameters in the UZL analysis information system.
Summary overview of urine collection:
| Analyses that can only be performed with hydrochloric acid addition: | Analyses that can also be performed from acidified urine: | Analyses that can only be performed without addition : |
| Ammonium (alternatively thymol/paraffin oil) Calcium HIES Catecholamines Magnesium Metanephrine Oxalate Phosphate + Clearance VMS and HVS |
Citrate Cortisol Glucose Urea Potassium Creatinine + Clearance Sodium |
Albumin Chloride Delta aminolevulinic acid Uric acid Osmolality Pancreatic amylase pH Porphobilinogen Protein total Trace elements and lead (special vessel) Thiocyanate |
If analyses from urine with and without acid addition are necessary, the urine must be collected on two different days.
Rejection of orders and samples
In the following cases, the ordered analyses are not performed:
- Missing or discrepant sample identification
- Wrong sample material
- blood stained vacutainer
- too long transport time or wrong transport for analytes that are not stable.
- insufficient quantity in the test tube.
- for external senders: missing information about the patient, which is necessary for billing.