Clinical vascular research
Vessels connect our entire body. This is also how we understand our research: our working group has been pursuing this integrative approach for two decades now.
“Man is as old as his vessels.” (R. Virchow, 1821-1902)

Clinical vascular research team (from left to right): Dr. Thomas Haider, Dr. Delia Nebunu, Dr. Leonie Kreysing, Prof. Dr. Andreas Flammer, Anne-Marieke Vegter, Dr. Matthias Nägele, Prof. Dr. Isabella Sudano, Dr. Sander Trenson.
Vessels connect heart, brain and hand
We do not regard the heart as an isolated structure, but as a vital team player in a complex network of vessels that spans the entire body and connects all organs with each other. For us, clinical research is always teamwork at heart: heart failure and prevention specialists work closely with study coordinators and postdocs across the disciplines of physiology, endocrinology, rheumatology, ophthalmology and neurology.
However, the most important concern of our interdisciplinary clinical research team remains the good and professional relationship with our patients. Our test subjects rely on good treatment in every respect. Their willingness to participate in studies involving vascular function measurements, data submission, biomaterials and randomization to innovative therapies enables us to find relevant answers to open scientific questions – or to raise new questions.
The endothelium, an organ the size of a football field that lines the inside of all our blood vessels, is the central object of our research. Humans have large vessels (macrovessels), such as the brachial artery, and very small vessels (microvessels), such as the vessels in the back of the eye (retinal vessels). The core of our research is to combine gold standard methods of vascular function measurement with new and innovative approaches. This opens up new perspectives on the different sections of our vascular bed and allows us to explore their role in relation to health, risk, disease and therapy.
Measurement of vascular function
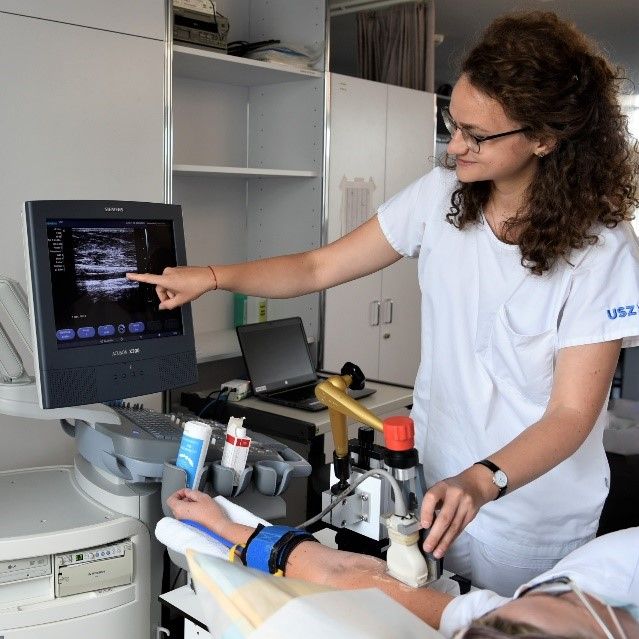
Endothelial function can now be examined clinically in several ways and has become a potentially important parameter for the early detection of arteriosclerotic vascular changes and for estimating the risk of cardiovascular disease later in life. The measurement of endothelium-dependent vasodilatation can be invasive and non-invasive. Invasive methods include, for example, intracoronary infusion of acetylcholine followed by quantitative coronary angiography. Non-invasive methods in particular have practical potential outside the catheter laboratory: Flow-mediated vasodilation (FMD) of the brachial artery (Fig. 2) , skin microcirculation using Doppler laser and, in particular, retinal vessel analysis (RVA, Fig. 1.).
We assess vessels structurally, e.g. with pulse wave analysis (PWA; Fig. 3) as a measure of arterial stiffness and with ocular fundus assessment as part of RVA (arterio-venous vascular ratio, AVR). Sonographic assessment of the intima-media thickness of the carotid arteries is also used for comprehensive phenotyping of vascular function.

The eye as a window to the heart
The dynamic retinal vessel analysis of the RVA allows us completely new insights in an almost astonishingly simple way: the eye as a window to the heart (Fig. 4).
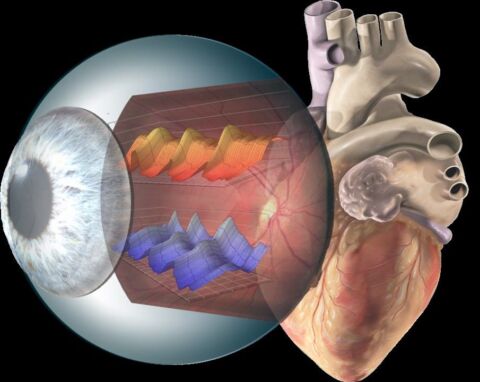
Fig. 4: Illustration of dynamic retinal vessel analysis.
The method is based on high-resolution video recordings with computer-based measurement of the blood vessels at the back of the eye before and after the application of flicker light. The flickering light increases the oxygen demand of the retina, which is compensated for by dilation of the associated vessels. This dilation is largely mediated by nitric oxide (NO), a central messenger substance of healthy endothelial cells. Insufficient dilation of the retinal vessels is therefore a sign of endothelial dysfunction of the small vessels (microvascular dysfunction).
The Clinical Vascular Research team is currently using DVA to investigate endothelial function in heart failure and classic risk factors. In an observational study, endothelial function in the small vessels in the eye is compared with endothelial function in the upper arm and vascular stiffness in patients with heart failure, subjects with cardiovascular risk factors and healthy controls. We were able to demonstrate microvascular damage in heart failure for the first time using dynamic functional analysis of retinal vessels and publish it at a high level (Nagele, Barthelmes et al. 2018). We were able to identify hypercholesterolemia as an important risk factor that can lead to microvascular dysfunction (Nagele, Barthelmes et al. 2018). It has also been shown that retinal microvascular dysfunction progressively increases during the progression of coronary heart disease to ischemic cardiomyopathy (Barthelmes, Nagele et al. 2019). Thus, retinal vascular function reflects the continuum of progressive vascular damage. As a result, the method could be very helpful for the characterization of different heart diseases, initial risk stratification and for assessing the success of therapy in patients with cardiovascular risk factors or diseases. As part of the ongoing observational study, we are therefore currently taking a more detailed look at the vascular profile of heart failure patients with preserved systolic ventricular function (HFpEF), for example.
Medication can improve – or worsen – vascular function
One focus of the research group is testing the cardiac safety of frequently prescribed drugs. Among other things, the effect of paracetamol, a commonly used painkiller, on blood pressure and endothelial function in patients with coronary heart disease was investigated. It was shown that paracetamol is not without cardiovascular side effects as assumed, but can increase blood pressure (Sudano, Flammer et al. 2010). Furthermore, the effects of polyphenol-rich substances (dark chocolate, pycnogenol) on endothelial function in patients with heart failure or coronary heart disease on vascular function were investigated. We were able to show that both pycnogenol and black chocolate can improve endothelial function in these patients and thus presumably counteract the development of atherosclerosis (Flammer, Hermann et al. 2007).
The team is currently investigating the role of endothelial function as a potential marker for the success of new heart failure therapies. In this context, we are currently investigating vascular function under valsartan/sacubtril (Entresto®) versus valsartan alone (VASCEND-LCZ). A recommendation for valsartan/sacubitril has already been included in the guidelines due to the clear improvement in prognosis and rehospitalization rates. We help to explain why valsartan/sacubitril is so promising. In addition to new drugs, modern medicine is increasingly using non-drug therapies. Cardiac resynchronization therapy (CRT) can significantly improve heart failure. The support from the SNSF enables us to investigate the effect of this cardiac resynchronization therapy on vascular function in more detail.
In addition to vascular function, another SNSF-funded project is focusing on sympathetic nerve activity. The aim of the OPIOVASC study is to investigate the vascular and sympathicotonic effects of opioids and non-steroidal anti-inflammatory drugs (NSAIDs) in patients with osteoarthritis compared to healthy subjects. We have already used this combination of measurement methods in previous studies. We were able to show that patients with Takotsubo syndrome had impaired endothelial function and increased sympathetic nervous system activity compared to the control group. This study underlines the potentially central role of stress and stress processing in the development of this disease (Naegele, Flammer et al. 2016).
Chocolate – dark chocolate – helps against stress. After our results on the benefits of dark chocolate on vascular function (Flammer, Hermann et al. 2007) were published with some media attention, we are currently continuing the investigation of polyphenol-like substances in a catheter study. We are testing the effect of a flavonoid drink (contained in dark chocolate) on the coronary arteries in patients who already have heart disease.
Blood volume as an important link in the cardiovascular system
Blood is the transport medium within the vascular network of our body and transports vital oxygen, nutrients and messenger substances (hormones) as well as metabolic end products and cells of the immune system and coagulation. The average human blood volume is about 5 liters. It is made up of approx. 60% fluid, the blood plasma, and approx. 40% solid components, mainly blood cells. It is regulated by various organs and hormone systems such as the renin-angiotensin-aldosterone system (RAAS). Diseases of the cardiovascular system such as cardiac insufficiency can lead to a pathologically altered blood volume status. Volume overload (hypervolemia) is the most common cause of decompensation of the cardiovascular system in heart failure and often leads to hospitalization and the use of diuretics. Conversely, anemia, a reduction in red blood cell mass, is observed relatively frequently in cardiac insufficiency. Anemia is associated with an increased risk of illness and increased mortality. Maintaining a physiological blood volume (euvolemia) is therefore of central importance in the treatment of heart failure. Indirect methods for determining the volume status, such as clinical signs of volume overload (e.g. neck vein congestion signs), are relatively imprecise and unspecific in this respect. The exact and reliable determination of blood volume using quantitative measurement methods therefore plays an essential role. The measurement of blood volume using the carbon monoxide rebreathing method (CORB, Fig. 5) is a non-invasive, safe and easy-to-use method for determining blood volume and its components (e.g. plasma volume, total hemoglobin mass, red blood cell volume). Using this method, we were recently able to show that heart failure patients with preserved left ventricular ejection fraction (HFpEF) can have a reduced blood volume compared to healthy individuals of the same age, which was reflected, among other things, in increased activity of the blood volume-regulating hormone systems (Montero, Haider et al. 2019). The reduced blood volume was due to a reduced red blood cell volume (RBCV), which can contribute to a reduced oxygen transport capacity with reduced physical performance. In a further comparative study, we were also able to show that even a single hemodialysis session leads to an altered response of the small vessels of the ocular fundus (especially the venules) to flicker light (Montero, Haider et al. 2020). In a prospective observational study (BLOVO-CVD1), we are currently investigating blood volume status and its role in vascular function in heart failure patients compared to patients with cardiovascular risk factors and healthy individuals of the same age. We are also investigating the role of blood volume in patients with treatment-resistant hypertension as part of an interdisciplinary collaborative project(HYRENE). In the future, we also want to increasingly evaluate the blood volume status in acute heart failure with the ambitious goal of establishing blood volume measurement for determining the current volume status and for monitoring and controlling dehydration therapy in the clinical routine of heart failure. In addition, we will also investigate the effects of new volume-regulating drugs such as SGLT2 inhibitors on volume and vascular status in clinically stabilized heart failure patients following acute decompensated heart failure (ADHF) as part of the DAPA-VOLVO project.
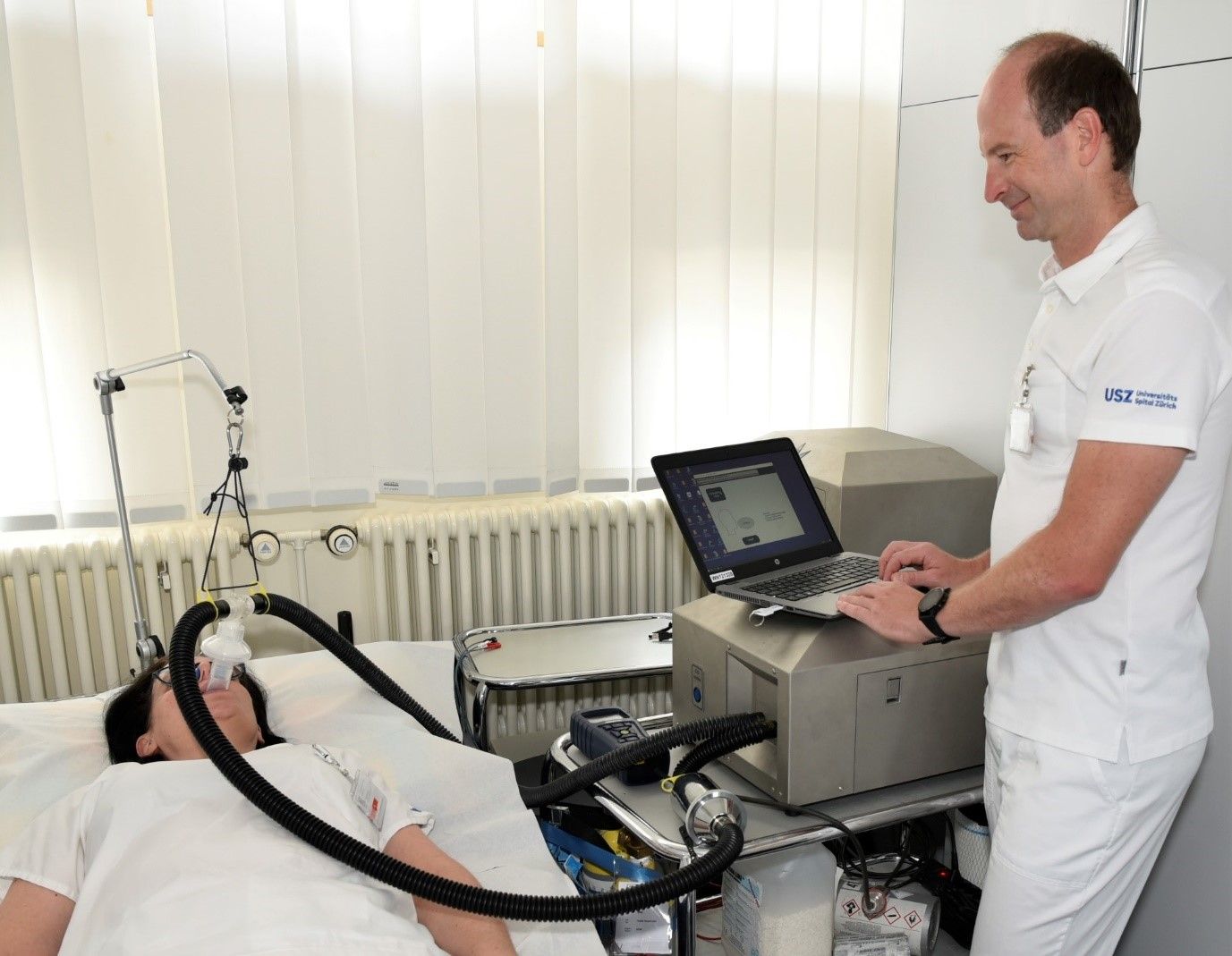
Fig. 5: Non-invasive measurement of blood volume using the carbon monoxide rebreathing (CO-RB) method and the use of a fully automated measuring device (OpCO, Detalo Health).
- Barthelmes, J., M. P. Nagele, S. Cantatore, E. Novruzov, V. Ludovici, A. von Eckardstein, M. Frank, F. Ruschitzka, I. Sudano and A. J. Flammer (2019). “Retinal microvascular dysfunction in patients with coronary artery disease with and without heart failure: a continuum?” Eur J Heart Fail 21(8): 988-997.
- Flammer, A. J., F. Hermann, I. Sudano, L. Spieker, M. Hermann, K. A. Cooper, M. Serafini, T. F. Luscher, F. Ruschitzka, G. Noll and R. Corti (2007). “Dark chocolate improves coronary vasomotion and reduces platelet reactivity.” Circulation 116(21): 2376-2382.
- Montero, D., T. Haider, J. Barthelmes, J. P. Goetze, S. Cantatore, I. Sudano, F. Ruschitzka and A. J. Flammer (2019). “Hypovolemia and reduced hemoglobin mass in patients with heart failure and preserved ejection fraction.” Physiol Rep 7(21): e14222.
- Montero, D., T. Haider, M. P. Nagele, J. Barthelmes, S. Cantatore, I. Sudano, F. Ruschitzka, M. Bonani and A. J. Flammer (2020). “Effects of hemodialysis on blood volume, macro- and microvascular function.” Microvasc Res 129: 103958.
- Naegele, M., A. J. Flammer, F. Enseleit, S. Roas, M. Frank, A. Hirt, P. Kaiser, S. Cantatore, C. Templin, G. Frohlich, M. Romanens, T. F. Luscher, F. Ruschitzka, G. Noll and I. Sudano (2016). “Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome.” Int J Cardiol 224: 226-230.
- Nagele, M. P., J. Barthelmes, V. Ludovici, S. Cantatore, M. Frank, F. Ruschitzka, A. J. Flammer and I. Sudano (2018). “Retinal microvascular dysfunction in hypercholesterolemia.” J Clin Lipidol 12(6): 1523-1531 e1522.
- Nagele, M. P., J. Barthelmes, V. Ludovici, S. Cantatore, A. von Eckardstein, F. Enseleit, T. F. Luscher, F. Ruschitzka, I. Sudano and A. J. Flammer (2018). “Retinal microvascular dysfunction in heart failure.” Eur Heart J 39(1): 47-56.
- Sudano, I., A. J. Flammer, D. Periat, F. Enseleit, M. Hermann, M. Wolfrum, A. Hirt, P. Kaiser, D. Hurlimann, M. Neidhart, S. Gay, J. Holzmeister, J. Nussberger, P. Mocharla, U. Landmesser, S. R. Haile, R. Corti, P. M. Vanhoutte, T. F. Luscher, G. Noll and F. Ruschitzka (2010). “Acetaminophen increases blood pressure in patients with coronary artery disease.” Circulation 122(18): 1789-1796.