Research Group Chiara F Magnani
Keywords
Cancer Immunotherapy, Gene Transfer, Chimeric Antigen Receptor T cells
Summary & Mission statement
Our research focuses on immunotherapy and uses genetic engineering to unleash our immune system against cancer. Specifically, we aim to apply non-viral engineering to design next-generation CAR T cells.
Overview
Chimeric antigen receptor (CAR) T cell immunotherapy has resulted in complete remission and durable response in highly refractory patients. My research seeks to take advantage of non-viral T cell engineering to timely implement a flexible and sustainable platform for next-generation CAR T immunotherapy. Indeed, despite important clinical success generated by CAR T cell-mediated immunotherapy in hemato-oncological diseases, the emergence of cases of resistance to single-target CAR T cells, the complexity of manufacturing, and the tumor microenvironment have limited progress in the field. To face these hurdles, we are designing non-viral next-generation CAR T cells, capable of recognizing more targets on tumor cells and designed to be less prone to tumor microenvironment inhibition. Non-viral gene transfer technology enables stable transgene expression and increased DNA carrying capacity through a simplified process with reduced costs and increased biosafety. We demonstrated that non-viral vectors can be used to achieve treatment of patients with fulminant relapse in an academic study using donor-derived anti-CD19 CAR T cells generated with Sleeping Beauty (SB) transposon in B-cell acute lymphoblastic leukemia (B-ALL) patients relapsed after HSCT. This is the first study in Europe using CAR T cells engineenered with the SB system.
Publications
See Publications
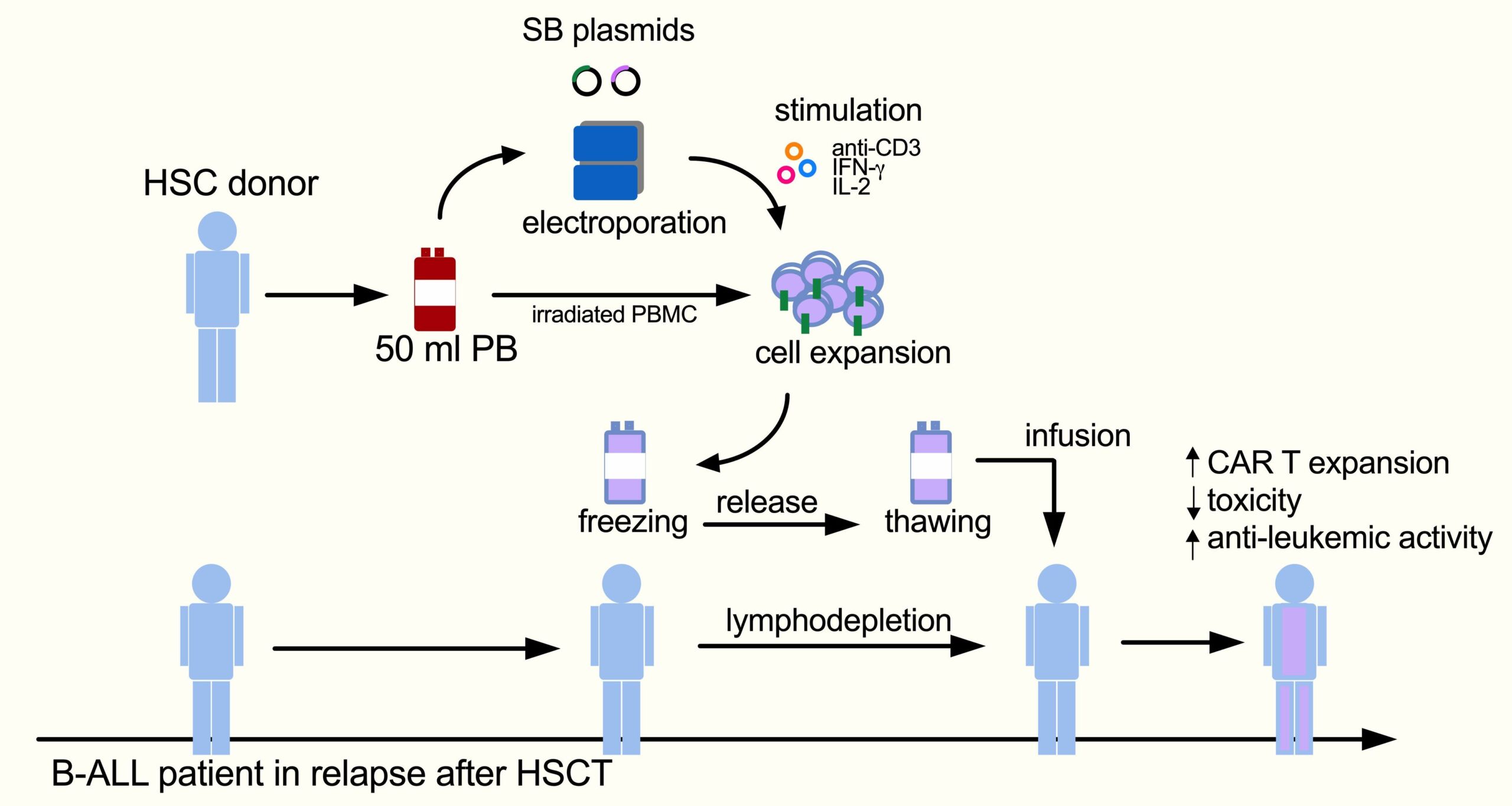
B-ALL patients, relapsed after transplantation, were treated with donor-derived anti-CD19 CAR T cells generated with SB. This study is an academic, multi-center, phase I/II dose-escalation trial enrolling pediatric and adult patients. After lymphodepletion with Fludarabine and Cyclophosphamide, patients are infused with a single dose of CAR T cells. CAR T cells are manufactured in-house from 50 ml of peripheral blood from the allo-transplant donor by electroporation with SB plasmids.
