Research Group Jana Ellegast
Keywords
Acute myeloid leukemia (AML), sarcoma, inflammatory signaling, target discovery, tumor environment
Summary
We study inflammation in cancer cells and their environment with a primary interest in leukemia and sarcoma, dissecting cancer biology to leverage inflammatory signaling in informed treatment strategies ultimately.
Mission statement
We aspire to exploit inflammatory signaling to discover mechanistically informed novel treatment strategies that improve the treatments and outcomes for patients with acute myeloid leukemia and sarcoma.
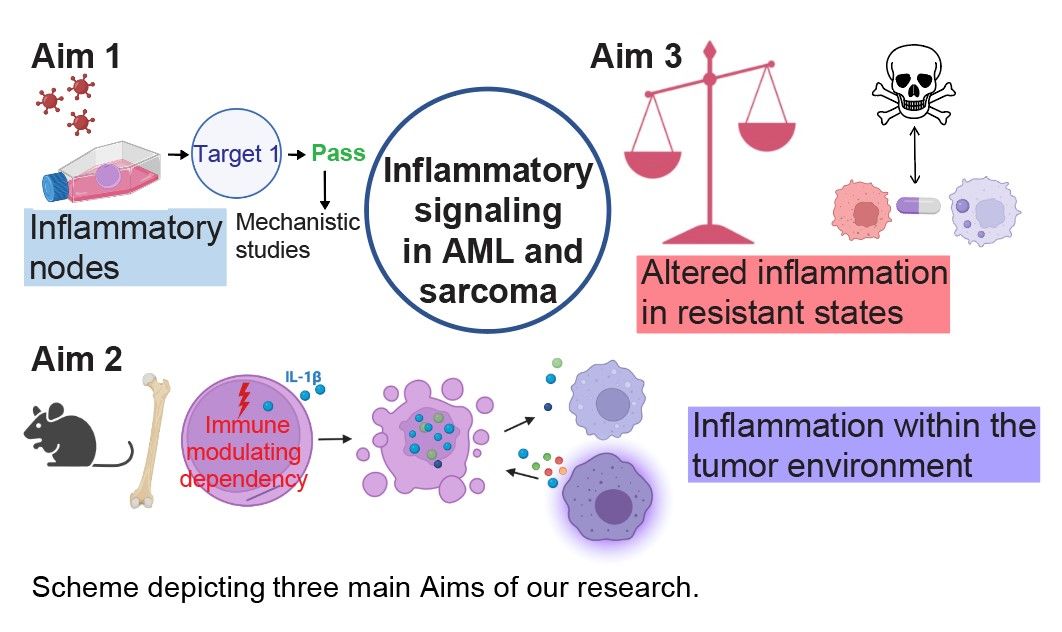
Overview
Dr. William Coley, considered the father of cancer immunotherapy, first injected a bone sarcoma with live streptococci over 130 years. Today, cancer immunotherapies have revolutionized treatment options for patients with numerous malignancies. Nevertheless, sarcoma and acute myeloid leukemia (AML) have not reaped the same benefits. Leukemia cells, however, are immune cells gone awry. In contrast to traditional immunotherapies, our research deciphers the role of cell-intrinsic inflammation: AML blasts depend on tight regulation of cell-intrinsic inflammation, and thus perturbation of this critical balance can be exploited as cell-intrinsic, self-directed immunotherapy (Ellegast et al. Cancer Discovery 2022). This discovery identifies acute hyper-inflammation as a vulnerability of leukemia cells, exemplifies its therapeutic potential, and guides future research. Deficient inflammation can also be detrimental to a leukemia cell, highlighting unbalanced inflammatory signaling as a key vulnerability in AML. While there is a need for more granular understanding to realize its translational potential, we have discovered and validated that some sarcoma types are equally susceptible to the perturbation of specific inflammatory pathways. My group’s research thus exploits inflammatory signaling as mechanistically informed transformative treatment strategies in AML and sarcoma.
Publications