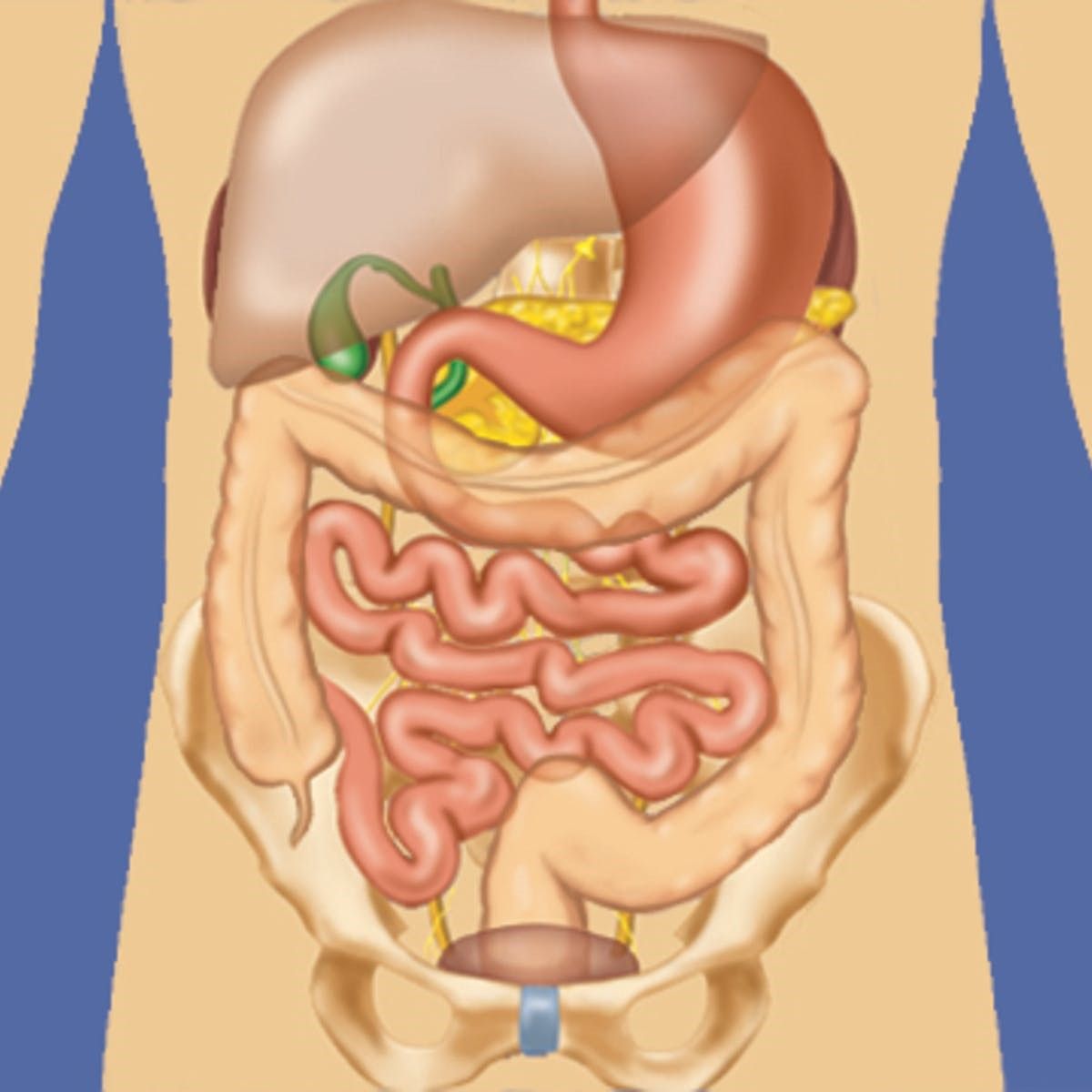
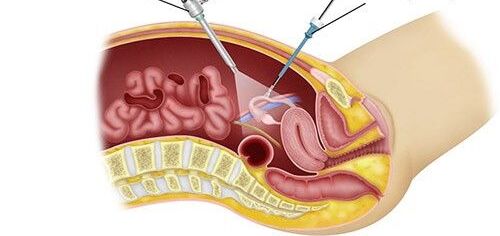
Overview
Peritoneal metastases are a secondary tumor manifestation of many tumors, for example of the appendix, colon, stomach or ovary. The clinical picture is caused by the tumor growing into the abdominal cavity, whereby individual tumor cells can spread freely. Primary peritoneal tumors, such as primary peritoneal mesothelioma, which originates from the surface of the peritoneum, are very rare. The range of primary tumor types and the additional histological subtypes present is large and therefore does not allow a generalization of treatment options and indications; the prognosis and thus the best treatment are very different and must be discussed at a tumor board. Each of our patients’ cases is discussed at the USZ’s interdisciplinary tumor board and the treatment concept is agreed with the referring physicians who have been caring for the patient to date.
Small metastases in the peritoneum in particular can be overlooked by imaging (CT, PET, MRI). Sometimes a laparoscopy is therefore advisable. The tumor is suspected in the following places:
Procedure for cytoreductive surgery and HIPEC
First, the abdominal cavity is opened and examined for metastases. The decision to have surgery is made if it can be performed radically (=completely). As part of cytoreductive surgery, all metastases in the abdomen are removed; sometimes it is also necessary to remove an organ. HIPEC then treats microscopic, remaining tumor cells and thus completes the result of the surgical procedure. The direct intra-abdominal application of cytostatic drugs achieves high concentrations in the abdominal cavity, while the exposure in the body remains low. Simultaneous heating of the chemotherapeutic carrier solution (dialysis fluid) improves the effect of the medication. The temperatures vary from 41° to 43° for 30-90 minutes, depending on the substances used. Intensive research is also being carried out in this area. Sometimes the procedure has to be aborted, for example if there is a pronounced tumor in the small intestine. Other treatment options are preferred in such cases.
What will I have operated on and removed?
If the decision is made to resect the tumor, the peritoneum is removed (peritonectomy) and the omentum (large mesh) is routinely removed. Organ resections are performed sparingly and only in cases of tumor infestation. The exception is oncological resections if the primary tumor (e.g. in the colon or appendix) has not already been removed. Sometimes it may be necessary to insert a stoma (artificial bowel outlet). HIPEC is then performed. The exact extent of the operation can only be defined during the operation due to the small metastases that are not visible on imaging.
Planned/possible operation:
Exploration and decision as to whether the procedure can be performed
Removal of
- Omentum (large net)
- Primary tumor
- Peritoneum (tumor-infested)
- Small intestine, large intestine
- Rectum
- Gallbladder
- Spleen
- Stomach
- Uterus, ovaries
Creation of an artificial bowel outlet (stoma)
Planned HIPEC:
- Mitomycin / Doxorubicin for 60min at 43°C
- Cisplatin / Doxorubicin for 60min at 43°C
Side effects and complications
The risk of the procedure depends primarily on the extent of the cytoreduction. To keep the rate of complications low, we have drawn up checklists that apply before, during and after the operation. Although the procedure can be extensive and therefore risky, we were able to reduce the complication rate and mortality rate at the USZ to 8.1 % and < 2 % respectively thanks to good interdisciplinary cooperation and clearly defined perioperative standards.
Possible complications are
- Anastomotic insufficiency
- Reoperation
- Transfer to the intensive care unit
- Pleural effusion
- Fistula of the pancreas, bile
- Passenger disorder (ileus)
- Bleeding
- Infection
- Thrombosis, rarely pulmonary embolism
- Digestive problems (diarrhea, cramps, adhesions)
What comes after the operation?
As a rule, there is a short stay in the intensive care unit immediately after the operation. The next day, patients can be transferred to a monitoring ward or directly to the surgical ward. During approximately 14 days, patients are supported in the recovery process by nursing, physiotherapy, nutritional counseling and the medical team. If you would like rehabilitation afterwards, we will organize this for you.
Depending on the results of the tissue examination (histopathology), follow-up treatment with chemotherapy is possible. Despite the complete removal of the tumor, recurrences can occur. Therefore, tumor follow-up care is absolutely necessary in the further course of the disease.