J Immunother Cancer. 2022 Feb;10(2):e003465
Philipp Busenhart, Ana Montalban-Arques, Egle Katkeviciute, Yasser Morsy, Chiara van Passen, Larissa Hering, Kirstin Atrott, Silvia Lang, Jesus Francisco Glaus Garzon, Elisabeth Naschberger, Arndt Hartmann, Gerhard Rogler, Michael Stürzl, Marianne Rebecca Spalinger, Michael Scharl
*CCCZ Members in bold
Abstract
Background
Integrin αvβ6 is a heterodimeric cell surface protein whose cellular expression is determined by the availability of the integrin β6 subunit (ITGB6). It is expressed at very low levels in most organs during tissue homeostasis but shows highly upregulated expression during the process of tumorigenesis in many cancers of epithelial origin. Notably, enhanced expression of integrin αvβ6 is associated with aggressive disease and poor prognosis in numerous carcinoma entities. Integrin αvβ6 is one of the major physiological activators of transforming growth factor-β (TGF-β), which has been shown to inhibit the antitumor T-cell response and cause resistance to immunotherapy in mouse models of colorectal and mammary cancer. In this study, we investigated the effect of ITGB6 expression and antibody-mediated integrin αvβ6 inhibition on the tumor immune response in colorectal cancer.
Methods
Using orthotopic and heterotopic tumor cell injection, we assessed the effect of ITGB6 on tumor growth and tumor immune response in wild type mice, mice with defective TGF-β signaling, and mice treated with anti-integrin αvβ6 antibodies. To examine the effect of ITGB6 in human colorectal cancer, we analyzed RNAseq data from the colon adenocarcinoma dataset of The Cancer Genome Atlas (TCGA-COAD).
Results
We demonstrate that expression of ITGB6 is an immune evasion strategy in colorectal cancer, causing inhibition of the antitumor immune response and resistance to immune checkpoint blockade therapy by activating latent TGF-β. Antibody-mediated inhibition of integrin αvβ6 sparked a potent cytotoxic T-cell response and overcame resistance to programmed cell death protein 1 (PD-1) blockade therapy in ITGB6 expressing tumors, provoking a drastic increase in anti-PD-1 treatment efficacy. Further, we show that the majority of tumors in patients with colorectal cancer express sufficient ITGB6 to provoke inhibition of the cytotoxic T-cell response, indicating that most patients could benefit from integrin αvβ6 blockade therapy.
Conclusions
These findings propose inhibition of integrin αvβ6 as a promising new therapy for colorectal cancer, which blocks tumor-promoting TGF-β activation, prevents tumor exclusion of cytotoxic T-cells and enhances the efficacy of immune checkpoint blockade therapy.
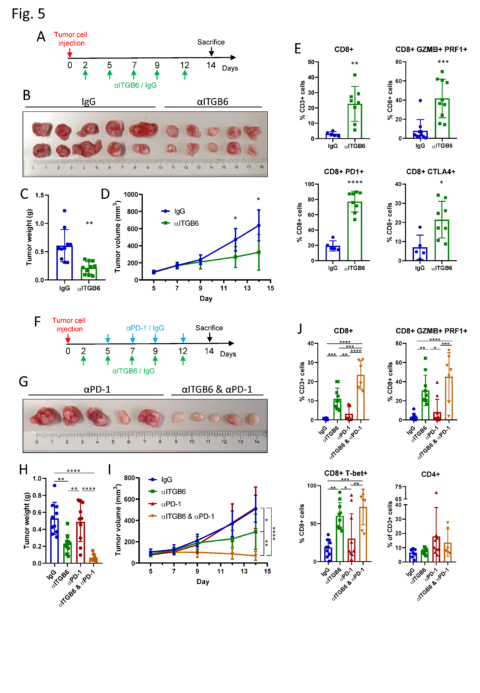
Integrin αvβ6 blockade sparks T-cell antitumor response and overcomes resistance to Checkpoint blockade therapy. (A) Experimental design of αITGB6 antibody (6.8G6) administration. (B) Subcutaneous CT26-ITGB6 tumors treated with αITGB6 or IgG control. (C) Tumor weight of subcutaneous CT26-ITGB6 tumors treated with αITGB6 or IgG control. (D) Tumor volume development of subcutaneous CT26-ITGB6 tumors treated with αITGB6 or IgG control. (E) Flow cytometry analysis of CD8+ Tcells in CT26-ITGB6 tumors treated with αITGB6 or IgG control. (F) Experimental design of αITGB6 (6.8G6) and αPD-1 antibody administration. (G) Representative image of subcutaneous CT26-ITGB6 tumors treated with αPD-1 or αITGB6 and αPD-1. (H) Tumor weight of subcutaneous CT26-ITGB6 tumors treated with αITGB6, αPD-1, αITGB6 and αPD-1 or IgG control. (I) Tumor volume development of subcutaneous CT26-ITGB6 tumors treated with αITGB6, αPD-1, αITGB6 and αPD-1 or IgG control. (J) Flow cytometry analysis of CD8+ Tcells in CT26-ITGB6 tumors treated with αITGB6, αPD-1, αITGB6 and αPD-1 or IgG control antibody. Means and SDs are shown (n=5 mice, 2 tumors per mouse). Unpaired two-tailed t-test (C, E) one-way analysis of variance (ANOVA) (H, J) and two way ANOVA (D and I) with Tukey’s post-hoc test were used to calculate statistical significance. *P<0.0001.

