Ann Surg. 2021 Nov 1;274(5):713-720.
Birrer DL, Golcher H, Casadei R, Haile SR, Fritsch R, Hussung S, Brunner TB, Fietkau R, Meyer T, Grützmann R, Merkel S, Ricci C, Ingaldi C, Di Marco M, Guido A, Serra C, Minni F, Pestalozzi B, Petrowsky H, DeOliveira M, Bechstein WO, Bruns CJ, Oberkofler CE, Puhan M, Lesurtel M, Heinrich S, Clavien PA.
*CCCZ Members in bold
Abstract
Objective:
The aim of this study was to pool data from randomized controlled trials (RCT) limited to resectable pancreatic ductal adenocarcinoma (PDAC) to determine whether a neoadjuvant therapy impacts on disease-free survival (DFS) and surgical outcome.
Summary Background Data:
Few underpowered studies have suggested benefits from neoadjuvant chemo (± radiation) for strictly resectable PDAC without offering conclusive recommendations.
Methods:
Three RCTs were identified comparing neoadjuvant chemo (± radio) therapy vs. upfront surgery followed by adjuvant therapy in all cases. Data were pooled targeting DFS as primary endpoint, whereas overall survival (OS), postoperative morbidity, and mortality were investigated as secondary endpoints. Survival endpoints DFS and OS were compared using Cox proportional hazards regression with study-specific baseline hazards.
Results:
A total of 130 patients were randomized (56 in the neoadjuvant and 74 in the control group). DFS was significantly longer in the neoadjuvant treatment group compared to surgery only [hazard ratio (HR) 0.6, 95% confidence interval (CI) 0.4–0.9] (P = 0.01). Furthermore, DFS for the subgroup of R0 resections was similarly longer in the neoadjuvant treated group (HR 0.6, 95% CI 0.35–0.9, P = 0.045). Although postoperative complications (Comprehensive Complication Index, CCI®) occurred less frequently (P = 0.008), patients after neoadjuvant therapy experienced a higher toxicity, but without negative impact on oncological or surgical outcome parameters.
Conclusion:
Neoadjuvant therapy can be offered as an acceptable standard of care for patients with purely resectable PDAC. Future research with the advances of precision oncology should now focus on the definition of the optimal regimen.
Ann Surg. 2021 Nov 1;274(5):713-720.
doi: 10.1097
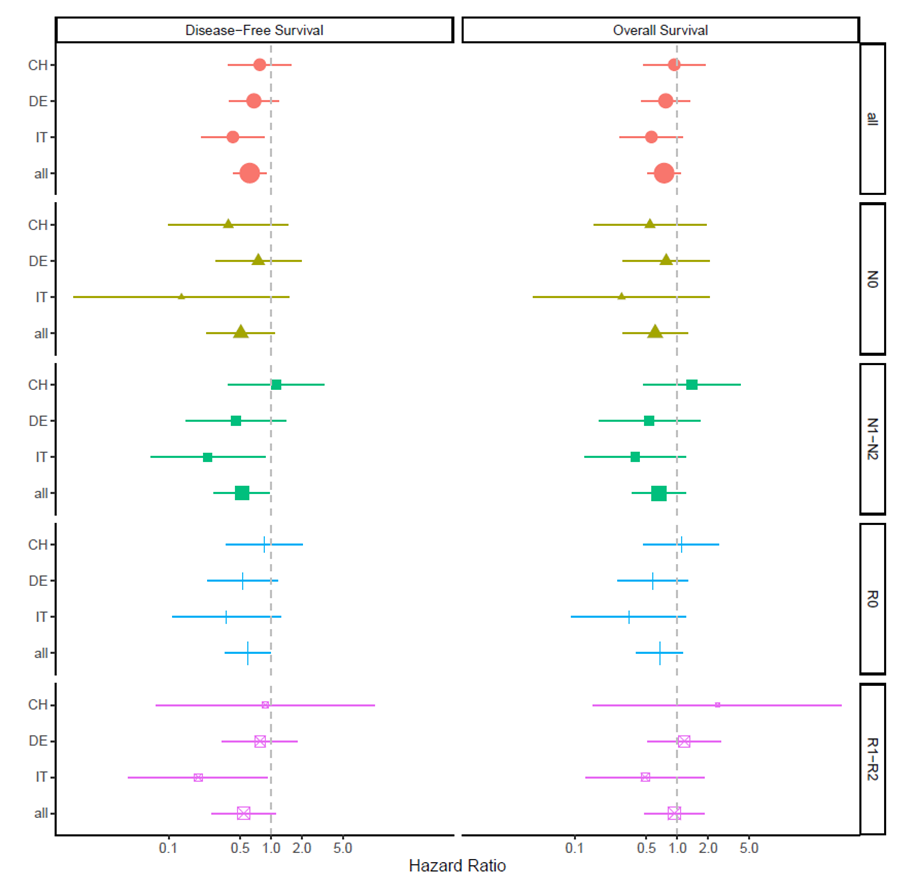
Patients who achieved a R0 resection (n = 69) revealed a significantly longer DFS following the neoadjuvant therapy compared to upfront surgery [HR 0.59 (95% CI: 0.35–0.99), P = 0.04]. Similarly, a better OS after R0 resection was observed in the neoadjuvant treated group, but the differences in survival were not statistically significant [HR 0.67 (0.40–1.13), P = 0.13]. In the subgroup of R1/2 resection, DFS appeared again longer following neoadjuvant therapy with 61% lower hazard for recurrence, but neither DFS [HR = 0.59 (0.29–1.20), P = 0.09] nor OS [HR = 0.97 (00.49–1.91), P = 0.84] was significant. Nodule negative subgroups do not display a significant difference, neither in DFS [HR 0.507 (0.236–1.00), P = 0.0812] nor OS [HR 0.607 (0.29–1.26) P = 0.182]. In the subgroup of nodule positive, the neoadjuvant group display a reduced hazard for recurrence or progression compared to the surgery group [HR 0.52 (0.28–0.97), P = 0.039]. OS was longer but not significant in the neoadjuvant treated group. [HR 0.66 (0.36–1.21), P = 0.182].

