Endocrine Tumors
Wir entwickeln Tumormodelle (in vitro und in vivo), etablieren neue nanotechnologische Therapieansätze und erforschen die Rolle des vault Komplexes in endokrinen Tumoren.
AG Hantel / Endokrine Tumore
Research Topics
Tumor models
As only a few cell lines are available for endocrine tumors these do not reflect heterogenous functional properties and specific therapeutic response rates of individual patients. To facilitate patient individual treatments and thereby to optimize therapeutic efficacy, we are aiming at the development and characterisation of patient-individual tumor models. Thus, the development of tumor models based on human surgical tumor material has been initiated. In the past, we were able to establish these types of in vivo tumor models for adrenocortical carcinoma and neuroendocrine tumors. Currently, we furthermore aim at 5D culturing of cell lines and primary cultures of endocrine tumors/ tissues.
In recent years it has been recognized that clinical translation of novel therapeutic strategies for patients with adrenocortical carcinoma (ACC) often fails. These disappointing results indicate that the currently utilized tumor models only poorly reflect relevant pathophysiology and, thereby, do not predict clinical applicability of novel pharmacological approaches. However, also the development of new preclinical ACC models has remained a challenge with only one human cell line (NCI-H295R) and one recently established human pediatric xenograft model (SJ-ACC3) being available for this highly heterogeneous malignancy. A recent study of our workgroup furthermore revealed a poor reproducibility of therapeutic action between different clones of the most commonly used tumor model NCI-H295R. In an attempt to broaden the current preclinical armamentarium, we aimed at the development of patient-individual tumor models. During these studies, one xenograft (MUC-1) displayed marked engraftment and sustained tumor growth. MUC-1 tumor analysis revealed highly vascularized, proliferating and SF-1 positive xenografts. In addition, we established a MUC-1 cell line with sustained nuclear SF-1 and cytoplasmic 3βHSD immuno-positivity.
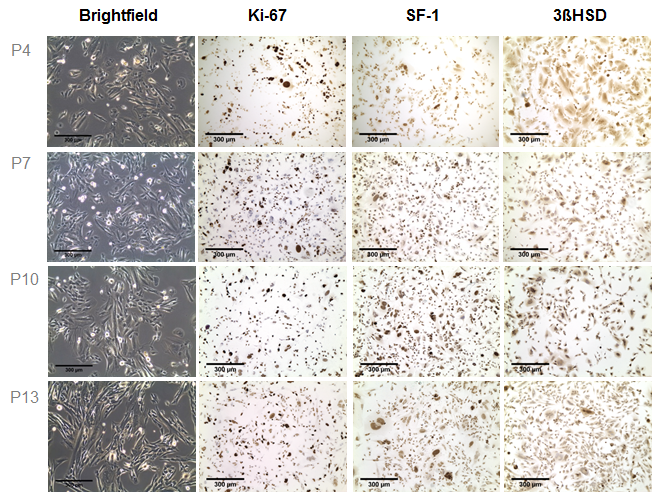
Of note, subsequent investigation of therapeutic responsiveness upon treatment with the current systemic gold standard EDP-M (etoposide, doxorubicin, cisplatin and mitotane) demonstrated maintenance of the clinically observed drug resistance for MUC-1 exclusively. The further characterization and implementation of MUC-1 is ongoing work in our lab.
Therapeutic approaches
In recent years the workgroup has focussed on the establishment of novel therapeutic strategies against endocrine tumors as as exemplified for an advanced chemotherapeutic, pathway specific and a combined approach below:
Liposomal EDP
We established novel therapeutic protocols for the treatment of adrenocortical carcinoma (ACC) patients by modification of existing polychemotherapies with nanotechnologically advanced cytostatic drugs. Starting point for this study was our observation of active internalization of liposomes by adrenocortical cells which indicated that liposomally encapsulated drugs could represent an interesting treatment option specifically advantageous for tumors of adrenocortical origin . Thus, we replaced the parental drugs of the clinical gold standard EDP-M (etoposide, doxorubicin, cisplatin, mitotane) by liposomal variants ( Study 1 & Study 2).
Signaling pathway specific
Activation of the Wnt/ß-catenin pathway is frequently observed in adrenocortical carcinoma (ACC) and has recently been shown to be associated with a more aggressive tumor phenotype. We have tested, in cooperation with the University of Paris novel therapeutic approaches by modulation of the Wnt-signaling pathway ( Study 1 & Study 2).
Hedgehog (Hh) signaling plays a crucial role during embryonic development, but is also involved in cancer development, progression, and metastasis. Patched, the receptor of the Hedgehog morphogen, is overexpressed in many cancer cells and the workgroup of our cooperation partners Dr. Lalli and Dr. Mus-Veteau (University of Nice) were recently able to provide a link between Patched and resistance to a variety of chemotherapeutic agents. Recently, we demonstrated together that Our results suggest that the use of an Inhibitor of Patched Drug efflux such as methiothepin in combination with doxorubicin could be a promising therapeutic option for adrenocortical carcinoma, and most likely also for other Patched-expressing cancers.
Immunoliposomes
In cooperation with the workgroup of Prof. Süss (University of Freiburg) we have furthermore developed a novel immunoliposomal approach by coupling a monoclonal IGF-1 receptor blocking antibody (1H7) to sterically stabilized liposomal doxorubicin (SSLD).
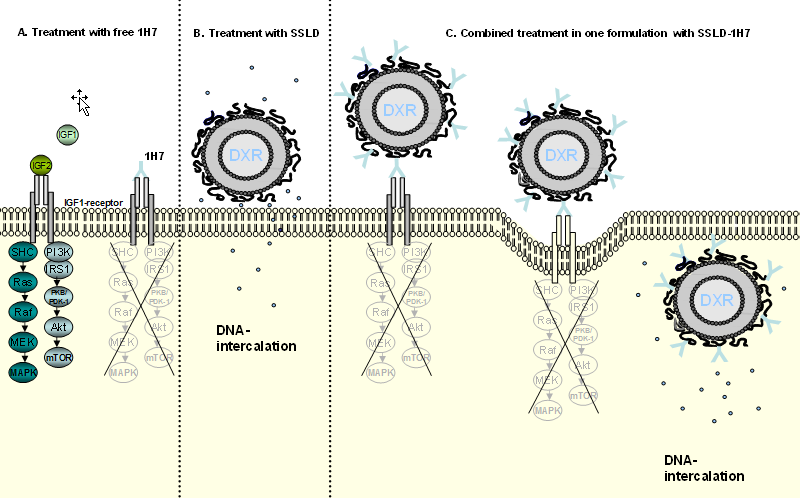
Such novel anti-IGFI-R immunoliposomes have been successfully tested in a preclinical model for human GEP-NETs. Moreover in vitro experiments indicated that usage of such a combinatory approach could also present a promising approach for other tumor entities such as breast cancer, neuroblastoma and prostate cancer and ACC (Study 1, Study 2, Study 3)
The Vault Complex
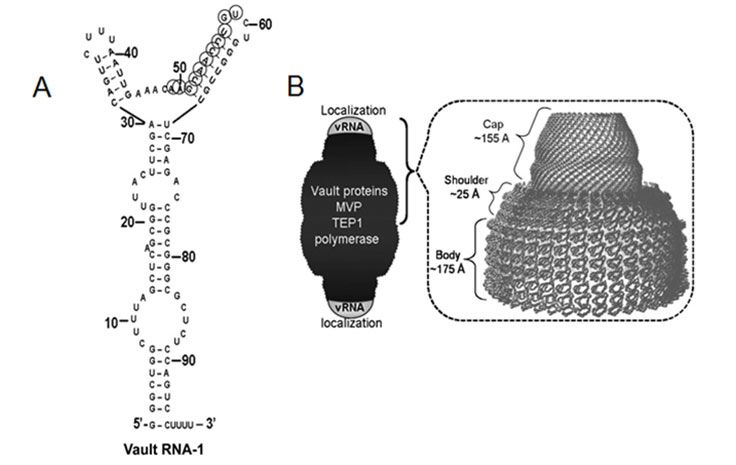 Recent findings from our workgroup indicate furthermore an unexpected role of the so called „vault complex“ in endocrine tumors. With a molecular mass of 12.9 MD, the vault complex outmatches by far other cellular particles like ribosomes in size and is thereby the largest ribonucleoprotein so far described. This barrel-shaped particle consists of multiple copies of the evolutionary highly conserved major vault protein (MVP, previously described as lung resistance-related protein LRP), two minor vault proteins (TEP1 and VPARP) as well as small non-coding RNA molecules (vault RNAs, figure: Gopinath et al 2010 Mol Canc Res ).
Recent findings from our workgroup indicate furthermore an unexpected role of the so called „vault complex“ in endocrine tumors. With a molecular mass of 12.9 MD, the vault complex outmatches by far other cellular particles like ribosomes in size and is thereby the largest ribonucleoprotein so far described. This barrel-shaped particle consists of multiple copies of the evolutionary highly conserved major vault protein (MVP, previously described as lung resistance-related protein LRP), two minor vault proteins (TEP1 and VPARP) as well as small non-coding RNA molecules (vault RNAs, figure: Gopinath et al 2010 Mol Canc Res ).
Cooperations
- Prof. Dr. Regine Süss, University of Freiburg, Germany
- Prof. Dr. Peter Igaz, University of Budapest, Hungary
- Prof. Dr. Norbert Polacek, University of Bern, Switzerland
- Prof. Dr. Stefan Bornstein, University of Dresden, Germany
- Prof. Dr. Martin Fassnacht, Prof. Dr. Stefanie Hahner, Dr. Silviu Sbiera, University of Würzburg, Germany
- PD Dr. Cristina Ronchi, University of Birmingham, UK
- Dr. Valentina Boeva, University of Paris, France
- Dr. Enzo Lalli and Dr. Isabelle Mus-Veteau. University of Nice, France
- Dr. Teni Boulikas, Regulon Inc., Athens, Greece
- Dr. Patrick Kugelmeier, Kugelmeiers, Zurich, Switzerland
Fundings
Uniscientia Foundation
German Research Foundation
Heuberg Foundation
Horizon 2020 – EU-FTI „Cells in Matrix“
Consortien
SFB/Transregio 205
HMZ Flagship Project – „Immuno-TargET“
