Radiobiology of Department of Radiation Oncology
The major focus of the preclinical research program of the Laboratory for Applied Radiobiology, Department of Radiation Oncology, University Hospital Zurich, is "Combined Treatment Modality". This program includes different aspects of translational research in the field of radiobiology investigating the cellular and tumoral response on the molecular, cellular and in vivo level to ionizing radiation alone and in combination with classical chemotherapeutical and novel pharmacological agents.
Founded in 1995 our laboratory is headed by Prof. Dr. Martin Pruschy, PhD and is completely integrated in both the preclinical research divisions of the head quarters of the University Hospital Zurich and in the clinical premises of the Department of Radiation Oncology.
It is this special environment that contributes to the translational spirit of our laboratory. Several PhD-candidates, MD- and PhD-Post-Doctoral fellows, and a highly motivated lab technician complete our team.
Research Program
The research activities consist of 3 major topics that investigate the cellular response to ionizing radiation alone and in combination with classical chemotherapeutical or novel pharmacological agents. A major goal is to understand treatment resistance on the molecular and cellular level and to translate novel combined treatment modalities into a clinical environment.
Zusammenfassung für Patientinnen und Patienten:
Dieses Projekt ist Teil unseres Forschungsschwerpunktes im Bereich der angewandten Krebsforschung zur Identifikation und Entwicklung neuer Radiochemotherapie Kombinations-modalitäten für Lungentumore. Unsere kürzlich erzielten Forschungsresultate zeigen auf, dass die Hemmung der plasmamembran-ständigen Matrixmetalloprotease ADAM17 Tumorzellen auf die Bestrahlung sensibilisiert. In diesem angewandten Forschungsprojekt wird die Rolle von ADAM17 anhand von klinischrelevanten Inhibitoren in humanem Tumormaterial (Lungentumorzellen und Tumorpartikel) und in Mäusetumormodellen für die Strahlensensibilisierung effizienz-und wirk mechanistisch genau untersucht. Dabei interessiert uns inwiefern Faktoren, welche von ADAM17 sezerniert werden, die interzelluläre Kommunikation und Strahlensensitivität beeinflussen. Gleichzeitig charakterisieren wir in Blutproben von Patientinnen und Patienten Faktoren, welche von dieser Metalloprotease unter Radiotherapie sezeniert wurden. Die Ergebnisse sind ausschlaggebend für die Lancierung klinischer Studien mit diesem neuen Konzept von ADAM17-Hemmung in Kombination mit Radiotherapie.
Project title:
Ionizing Radiation and Inhibition of ADAM17: Mechanistic and Translational Research Towards a Novel Combined Treatment Modality
Principle investigators:
Martin Pruschy, Laboratory for Applied Radiobiology, Dept. Radiation Oncology, USZ
Alex Soltermann, Dept. Pathology, USZ
Oliver Riesterer, Kantonsspital Aarau
Matthias Guckenberger, Dept. Radiation Oncology, USZ
Contributing researchers:
Philip Knobel, Fabienne Tschanz, Verena Waller, Tamara Kazimova, Rona Winkler
Funding:
SNF, KFS
Background:
The insult on the level of DNA is most important for the cytotoxicity of ionizing radiation. However, RT also induces a multilayered stress response in the tumor with para- and autocrine factors released into the tumor microenvironment, which co-determine the treatment response. ADAM17 is a membrane-associated metalloproteinase, which is actively associated with the process of proteolytic shedding of membrane-bound proteins and hence the rapid modulation of key signals in the tumor microenvironment. Increased ADAM17 expression in non-small cell lung cancer is associated with aggressive progression and poor prognosis. We recently provided novel insights into activation of ADAM17 and subsequent ligand shedding in response to irradiation and demonstrated that direct targeting of ADAM17 sensitizes lung carcinoma cells to RT.
Aims:
We aim to mechanistically investigate the role of (RT-induced) ADAM17-ligand shedding and inhibition of ADAM17 for radiosensitization in clinically relevant tumor models and to demonstrate that these RT-induced processes also occur in lung adenocarcinoma patient-derived material. We will probe the efficacy of ADAM17 targeting in combination with ionizing radiation in established and primary human lung adenocarcinoma cell lines with an orally bioavailable small-molecular inhibitor of ADAM17 and a novel ADAM17-directed inhibitory antibody. This treatment combination will also be investigated in state-of-the-art orthotopic lung tumor models
We focus on the analysis of a differential RT/ADAM17-dependent-secretome derived from established and primary tumor cell lines and in the serum of tumor carrying mice in response to RT/ADAM17-inhibition. Furthermore, we will investigate the treatment response in tumor slice cultures derived from human adenocarcinoma patients, which maintain their original tumor heterogeneity with an integrated stroma and tumor vasculature. We will mechanistically investigate the relevance of RT-induced ADAM17-activity and shed factors in genetically engineered human lung adenocarcinoma tumor cells with wildtype and downregulated ADAM17 and tumors thereof and aim to identify RT/ADAM17-regulated signal transduction cascades of relevance for the treatment response to ionizing radiation. In subsequent in vivo studies we will investigate RT/ADAM17- dependent modulation of the tumor microenvironment and will assess the role of RT/ADAM17-dependent intercellular communication for intrinsic and induced radiation resistance in vivo.
Research Output:
Several oral presentations at international conferences (ESTRO, Wolfsberg, EACR)
Key manuscript on first part submitted.
The matrix metalloprotease ADAM17 is shedding factors from tumor cells, that act in auto-and paracrine ways (tumor cells; tumor microenvironment; immune system) and thereby codetermine the radiation response of tumors.
Zusammenfassung für Patientinnen und Patienten:
Durch die Identifizierung von immunsuppressiven Mechanismen und der entsprechenden Entwicklung von Immun-Checkpoint-Inhibitoren stellt die Immuntherapie einen wichtigen Pfeiler der modernen Krebstherapie dar – sei es als Einzeltherapie oder in Kombination mit Chemo- und Radiotherapie. Im Rahmen dieses Projektes, welches vom Cancer Research Center der Universität Zürich/ETH-Zürich ausgezeichnet wurde, werden wir unsere Expertise im Bereich der experimentellen Radiotherapie und experimentellen Immunologie kombinieren, um die neue Kombinationsform von hIL/NARA1cs mit Radiotherapie in präklinischen Tumormodellen zu untersuchen. Desweiteren werden wir immunologisch-relevante Endpunkte in Blutproben bestrahlter Patientinnen und Patienten untersuchen. Dieses Projekt ist von grossem translationalen Interesse. Die Resultate werden entscheidend für den Start von klinischen Versuchen an Patientinnen und Patienten mit dieser neuen Kombinationsform sein.
Project title:
Alternative Combined Radioimmunotherapy to Overcome Treatment-Related Resistances
Principle investigators:
Martin Pruschy, Laboratory for Applied Radiobiology, Dept. Radiation Oncology, USZ
Onur Boyman, Immunology Research Laboratory, Dept. Immunology, USZ
Contributing researchers:
tbd, Dilara Sahin, Irma Telarovic, Fabienne Tschanz, Philip Knobel
Funding:
This project is supported by the Cancer Research Center, UZH, 2019-2020
Background:
Radiotherapy (RT) induces a potent immune response, which could be further exploited based on our mechanistic understanding of the interplay between immunostimulatory and immunosuppressive antitumor responses. This is supported by the promising preclinical and clinical results from the combined treatment modalities of RT with immune checkpoint inhibitors. However, unlike immune checkpoint inhibitors, IL-2 immunotherapy does not rely on the expression of immune checkpoint molecules by tumor cells. Thus, IL-2 immunotherapy might be applicable to a broader range of cancer types, particularly in combination with RT.
The anti-cancer properties of IL-2 are mediated by its capacity to stimulate CD8+ T and NK cells. This comes along with the stimulation of immunosuppressive Treg cells and systemic side effects. By using hIL-2/NARA1cx we avoid and significantly reduce IL-2’s effects on CD25+ cells, particularly Treg and endothelial cells. Moreover, hIL-2/NARA1cx preferentially stimulate effector immune cells and prolong the in vivo half-life of IL-2, thus resulting in favorable anti-tumor immune responses in various pre-clinical cancer models.
Aims:
Our research program has the following overarching goals:
i) To develop novel combined treatment modalities at the interface of RT-induced immunostimulatory and immunosuppressive processes.
ii) To develop novel combined treatment modalities augmenting the systemic abscopal effect of RT.
In particular we will probe the combined treatment modality of radiotherapy (RT) with hIL-2/NARA1 complexes (hIL-2/NARA1cx) in lung and melanoma tumor models. We will probe the abscopal effect on RT and hIL-2/NARA1cx combination therapy and will analysis the secretome toward the identification of biomarkers of interest
Research Output:
The specific project will only start in 2019
Zusammenfassung für Patientinnen und Patienten:
Die Bestrahlung von Tumoren mit Partikeln (z.B. Protonen) erlaubt das Aussparen von gesundem Gewebe bei ähnlicher bis verbesserter Tumorkontrolle wobei die zellbiologischen Vorgänge nach der Partikelbestrahlung weitestgehend unbekannt sind. In diesem Forschungsprojekt, welches die Radiobiologie-Gruppe der Klinik für Radioonkologie zusammen mit dem Paul-Scherrer-Institut (Villigen, CH) und dem MedAustron (Wiener Neustadt, A) durchführt, möchten wir herausfinden, ob, abhängig von Protonen- bzw. Photonenbestrahlung (Röntgenstrahlung), Unterschiede in den DNA-Reparaturmechanismen auftreten. Hierfür wird das Ansprechen der Tumorzellen auf die verschiedenen Strahlenarten gemessen und verschiedene Immunofluorezenz-Färbungen durchgeführt, um die DNA-Reparatur nachverfolgen zu können. Weiters wird eine Kombination mit einem Chemotherapeutikum getestet, um Zellen speziell für Protonenbestrahlung zu sensibilisieren. Erkenntnisse würden es erlauben, das Ansprechen eines Patienten oder einer Patientin auf Protonenbestrahlung abzuschätzen beziehungsweise Protonenbestrahlung mit einer Chemotherapie zu kombinieren, um das klinische Anprechen zu verbessern.
Project title:
Elucidating the role of DNA double strand break repair machineries as predictor for proton radiotherapy outcome and its modulation by established chemotherapeutics
Principle investigators:
Martin Pruschy, Department for Radiation Oncology, USZ
Tony Lomax, PSI, Villigen
Contributing researchers:
Simon Deycmar, Department for Radiation Oncology, USZ
Silvia Gruber, MedAustron
Funding:
This project is supported by the SBFI No. 15.0066 and part of a Horizon2020 research and innovation program under the Marie-Sklodowska-Curie grant agreement No. 642623
Background:
Radiotherapy is a major treatment option in almost all solid tumor entities with external photon irradiation as most common radiotherapy form. Driven by its superior dose-distribution profile allowing to spare healthy tissue from severe side effects, particle-based radiotherapy is stepping out of its niche. Despite our growing knowledge regarding different physics, the different biological and cellular response to particle irradiation remains widely unexplored. Recent findings by the Laboratory for Applied Radiobiology, Dept. Radiation Oncology, University Hospital Zurich (headed by Prof. Dr. M. Pruschy) demonstrated a differential dependence on the two key DNA double strand break (DSB) repair machineries subsequent to photon and proton irradiation, respectively. These findings were supported by independent results of the Willers’s group at the Massachusetts General Hospital. Thus, DSB repair capacity could become a predictor for proton radiotherapy outcome as well as a rational for combined chemoradiotherapy concepts.
In this project, we are interested to take a leap forward and convert mentioned findings regarding the differential deployment of the key DSB repair machineries from basic research to a translational setting. Proton radiotherapy will be administered in a spread-out Bragg peak in scanning mode, mimicking the currently most advanced clinical proton treatment conducted. Subsequent to irradiation, we will follow-up emerging nuclear repair foci by immunofluorescence to elucidate the kinetics of repair foci assembly, removal and the co-localization of repair proteins. Clonogenic assays will enable us to link repair deficiencies with the susceptibility of the irradiated cells and provide us with a clinically employable in vitro readout. Promising results from our own research demonstrate a proton-specific radiosensitization by ganetespib, which demands to be underpinned by prementioned assays to understand the mechanism behind and to enable a future conversion into clinics.
Aims:
The role and differences of DNA double strand break repair subsequent to proton or photon radiotherapy shall be elucidated to enable a rational patient stratification including biological parameters, respectively combination with clinically relevant chemotherapeutics.
Research Output:
• PhD Thesis – Simon Deycmar
• Publication
• Oral Presentation at the “58th annual conference of the Particle Therapy Co-Operative Group (PTCOG)” in Manchester/UK, 10-15.6.2019
Zusammenfassung für Patientinnen und Patienten:
Radiotherapie wirkt toxisch gegen den bestrahlten Tumor, führt aber auch zu Normalgewebsschäden in mitbestrahlten gesunden Organen. Das Volumen des mitbestrahlten Gewebes ist mitentscheidend, wie gravierend diese Normalgewebsschäden sich auswirken. Zirkulierende Blutzellen und damit einhergehende Zellen des Immunsystemes werden ebenfalls mitbestrahlt, doch wir kennen zur Zeit kaum die Auswirkungen eines unterschiedlichen Bestrahlungsvolumens auf die Immunantwort gegen den bestrahlten Tumor. In diesem Projekt untersuchen wir den Einfluss des Bestrahlungsvolumen auf die Immunantwort in klinisch-relevanten Maustumormodellen.
Project title:
Radiotherapy treatment volume and its role for the tumor-oriented immune response
Principle investigators:
Martin Pruschy, Laboratory for Applied Radiobiology, Dept. Radiation Oncology, USZ
Jan Unkelbach, Medical Physics Research Group, Dept. Radiation Oncology, USZ
in collaboration with: Onur Boyman, Immunology Research Laboratory, Dept. Immunology, USZ
Contributing researchers:
Irma Telarovic, Ivo Grgic, Jérôme Krayenbuehl
Funding:
Cancer League Zurich
Background:
Despite continuous multidisciplinary efforts, the prospect to transform advanced tumors into a state of “chronic disease” is still limited. Tumor immune evasion and associated failure of immunotherapy are contributing reasons for this and are based on insufficient infiltration or effectiveness of immune competent cells in the tumor. Radiotherapy has been demonstrated to overcome the immunosuppressive tumor microenvironment and anecdotal reports suggest that local tumor irradiation may even exert systemic or abscopal anti-tumor effects by immune-response modification with subsequent response of non-irradiated tumor metastases. Radiotherapy acts cytotoxic against the tumor and the co-irradiated tumor (micro)environment. Thus, radiotherapy has detrimental effects not only on tumor infiltrating lymphocytes but also on co-irradiated circulating lymphocytes in the bloodstream during radiotherapy, as these cells are particularly radiosensitive. Prolonged lymphopenia during or after the tumor treatment has been shown to be a prognostic factor for the overall survival in many cancer types and the role of radiotherapy in this respect may be suspected. Thereby, larger radiotherapy treatment volumes may lead to a decreased immunogenic effect of a combined immunoradiotherapy and might also suppress abscopal effects, which are indeed only rarely observed in the clinic. Furthermore, large elective target volumes (e.g. elective lymph node irradiation) are often included into the radiotherapy treatment plan to eradicate potential microscopic tumor cells. In the setting of abscopal or systemic immune responses, this may be unnecessary or even harmful regarding irradiated immune cells.
Aims:
To investigate the impact of the radiotherapy treatment volume on the efficacy and the immune response alone and in combination with immune checkpoint inhibitors against the irradiated primary tumor and abscopal tumor burdens. We will use established preclinical murine tumor models and a state-of-the art small animal image-guided radiotherapy platform for exact irradiation of the treatment volume. Smaller treatment volumes may decrease the number of co-irradiated (circulating) lymphocytes substantially, which may lead to a significantly increased immune response towards the primary, but also the non-irradiated secondary tumor. Larger treatment volumes may diminish immune responses below a critical threshold for an effective immune response. In extremis, even just partial primary tumor irradiation might elicit a sufficiently strong immune response.
Research Output:
ESTRO Abstract 2019:
• Probing spatiotemporal fractionation of the preclinical level. Irma Telarovic, Jan Unkelbach, Ivo Grgic, Jerome Krayenbuehl, Matthias Guckenberger, Martin Pruschy.

Figure 1: We will use established preclinical murine tumor models to test the impact of the radiotherapy treatment volume in a single- and a double-tumor model (left). A state-of-the-art small animal image-guided radiotherapy treatment platform will be used for the precise volume irradiation (middle). Further investigation will include tumor growth delay experiments (determined by both caliper and CT volumetry) and the tumor microenvironment- and circulating blood-related endpoints (right).
Zusammenfassung für Patientinnen und Patienten:
Wir sind an der Identifizierung von Proteinen interessiert, die eine Wechselwirkung mit dem Protein ADAM17 im Tumor-Mikromilieu eingehen, und deren Hemmung die Bestrahlungsempfindlichkeit weiter erhöht.
Zusätzlich sind wir an der Identifizierung von Proteinen interessiert, die in Wechselwirkung mit ADAM17 stehen und einen parakrinen stimulierenden Effekt auf distale, sekundäre Tumore und Metastasen haben, und deren Hemmung die Bildung von sekundären, distalen Tumoren und Metastase verhindert. Dazu werden wir die sogenannte BirA-Markierungs-Technik anwenden, die uns erlaubt, Proteine, die in unmittelbarer Wechselwirkung mit ADAM17 stehen, über ein BirA-Enzym zu markieren und zu identifizieren. Diese neuartige Methode hat gegenüber Standarttechniken für die Identifizierung von Protein-Wechselwirkungen wie die Immunpräzipitation folgende Vorteile.
1) Anwendbar in Zellkulturen sowie in Mäusen.
2) Die Markierung von Proteinen erfolgt über einen bestimmten Zeitraum. Dadurch werden auch schnelle und instabile Wechselwirkungen identifiziert.
Project title:
Exploring the interactome of ADAM17 in the tumor microenvironment and its role for radiation resistance
Principle investigators:
Philip Knobel, Department for Radiation Oncology, USZ
Martin Pruschy, Department for Radiation Oncology, USZ
Contributing researchers:
Verena Waller, Department for Radiation Oncology, USZ
Funding:
This project is supported by the Vontobel Stiftung, Krebsliga Zürich, the Stiftung zur Krebsbekämpfung and Stiftung für angewandte Krebsforschung
Background:
In addition to DNA damage and genomic instability, ionizing irradiation (IR) also affects intra- and intercellular processes that induce a multilayered stress response and determine the tumor response to radiotherapy. Growth and survival of NSCLC cells are often dependent on ectodomain shedding, which includes proteolytic cleavage of the extracellular part of membrane proteins primarily mediated by membrane-anchored metalloproteases. Most of the members of the ADAM (A disintegrin and metalloproteinase) family have proteolytic activity and are actively associated with the process of proteolytic ‘shedding’ of membrane-bound proteins and hence the rapid modulation of key signals in the tumor microenvironment. ADAM17 represents one of the best studied members of the ADAM family. In patients suffering from NSCLC increased ADAM17 expression is associated with aggressive progression and poor prognosis. ADAM17 drives pleiotropic pathways that are involved in auto- and paracrine signaling by regulating the processing of multiple key oncogenic growth factors, cytokines and adhesion molecules.
As part of an IR-dependent secretome analysis, our laboratory demonstrated that adenocarcinoma cells respond to IR with elevated ADAM17 activity that negatively influences the outcome of non-small-cell-lung-cancer (NSCLC) to the treatment. Direct targeting and inhibition of ADAM17 sensitizes multiple adenocarcinoma cell lines to radiotherapy in vitro and in vivo, which makes it a promising target for novel combined treatment modalities.
Aims:
We are highly interested in gaining additional insights into the mechanisms of IR-induced and ADAM17-mediated modulation of key signals in the tumor microenvironment. We aim to identify substrates of ADAM17 in the primary tumor or metastatic tumor sites that could be targeted to increase the sensitivity of the tumor towards radiotherapy.
Methods:
The novel BioID method will be applied to identify ADAM17 proximity interactors and to determine how ADAM17 influences its microenvironment. The principle of the BioID method is based on proximity-dependent biotin labeling of primary amines of proteins by a promiscuous Escherichia coli biotin protein ligase (BirA), fused to either the C- or N-terminus of a protein of interest. Biotinylated proteins are purified via streptavidin and identified by mass spectrometry (Figure 1). For this project, BirA was fused to the extracellular N-terminus of murine ADAM17 and overexpressed in multiple murine and human lung cancer cell lines. Promising candidates identified by mass spectrometry (MS) will be tested in in vitro approaches and in vivo in immune competent orthotopic lung tumor models.
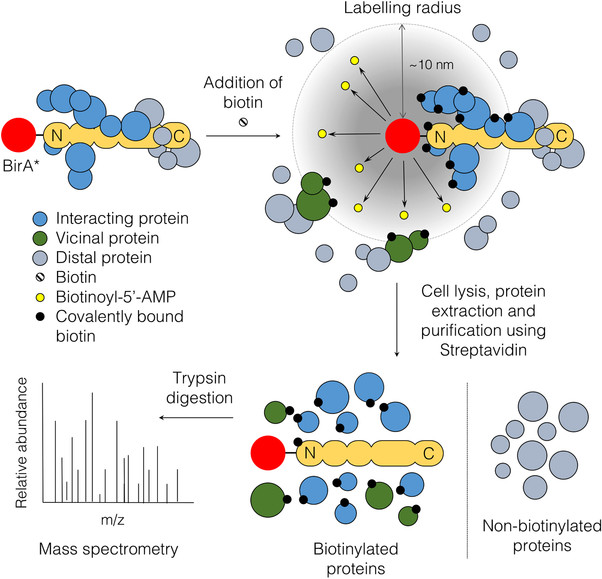
Figure 1: The principle of BioID. Expression of the fusion protein of interest with BirA*. Under biotin- and ATP-supplemented condition, the ligase synthesizes highly active biotinoyl-5’AMP (yellow) which can react with primary amines on proximal proteins within a labeling radius of 10 nm. Biotinylated proteins are purified via streptavidin pull-down and identified by mass spectrometry (Varnaite et al., 2016).