Basic and translational Research
Stem Cells & Osteology
Tissue engineering research has endeavored to search for novel sources of stem cells other than bone marrow mesenchymal stem cells (MSCs) for bone regeneration and repair. Fractures with a critical size bone defect (e.g. open fracture with segmental bone loss) are associated with high rates of delayed- and non-union. The prevention and treatment of these complications remain a serious issue in trauma and orthopaedic surgery. Autologous cancellous bone grafting is a well-established and widely used technique. However, it has some drawbacks related to availability, increased morbidity and insufficient efficacy. Mesenchymal stem cells (MSCs) can potentially be used to improve fracture healing. Particularly human fat tissue has been identified as a good source of multilineage adipose derived stem cells (ASCs), which can be differentiated into osteoblasts. The main issue is that MSCs are a heterogeneous population of progenitors and lineage-committed cells harboring a broad range of regenerative properties. This heterogeneity is also mirrored in the differentiation potential of these cells. In our studies we test the possibility to enrich defined subpopulations of stem/progenitor cells for direct therapeutic application without requiring an in vitro expansion. The most promising enriched stem cells populations are tested for their regeneration capacity in mouse models.
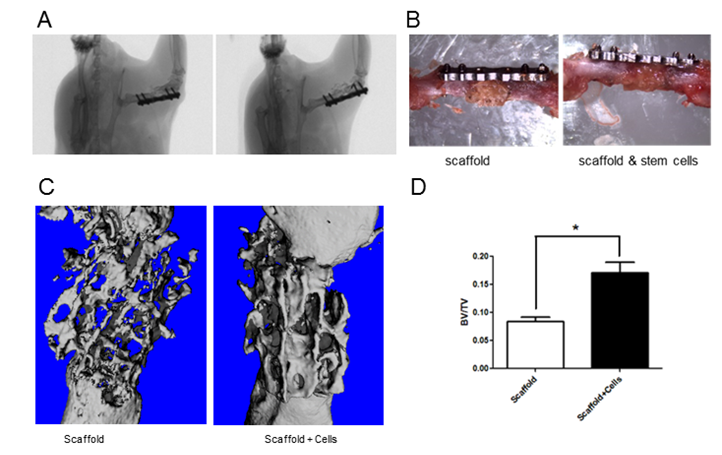
A. In vivo micro computed tomography picture of implanted scaffold. B. Difference between implanted scaffold and scaffold with enriched stem cells. C. microCT 3D reconstructions of collagenous bone scaffold with and without stem cells implanted in a femoral segmental critical-sized defect in mouse, 8 weeks upon implantation. D. Quantification of bone volume to total volume in the two groups with and without stem cells.
Stem cells self-renewal and differentiation
We are interested in the identification, establishment, characterization and analysis of stem cells, particularly in the study and implementation of their potential for therapeutic and medical applications.
Stem cells are a powerful tool not only for the study of biological processes, but also for their potential therapeutic application. One of the main issues with the use of stem cells for clinical applications is the ability to maintain these cells outside of the body (in vitro) in a self-renewing pluripotent and/or multipotent state and to precisely differentiate them to specific cell types. The mechanisms underlying maintenance and determination of pluripotency as well as the ones driving differentiation are nevertheless still largely unknown. Our laboratory has two main research focuses:
- Understanding the molecular mechanisms involved in the maintenance of stem cell identity and driving differentiation.
- Development and optimization of tissue engineering approaches for bone regeneration with multipotent and pluripotent stem cells.
We spent in the past years a lot of efforts in understanding the molecular mechanisms involved in the maintenance of stem cell identity in different stem cell populations (embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cell and neural crest stem cells). For these studies we are performing state of the art high throughput screenings at transcriptomic and proteomic levels and generating iPSCs through reprogramming from mouse and human somatic cells. In particular we are interested in optimizing the generation of patient specific iPSCs for the development of personalized medicine, like drug screening and tissue engineering. In the past years, we could identify factors important for the maintenance of pluripotency and in driving reprogramming of somatic cells. We could for example identify Pramel7 (preferentially expressed antigene in melanoma like 7) as a novel factor crucial self-renewal in ESCs (Casanova et al., Stem Cells 2011).and recently elucidate the role of role of poly-ADP-ribose polymerase 1 (Parp1) during reprogramming (Weber et al. Stem Cells, 2013).
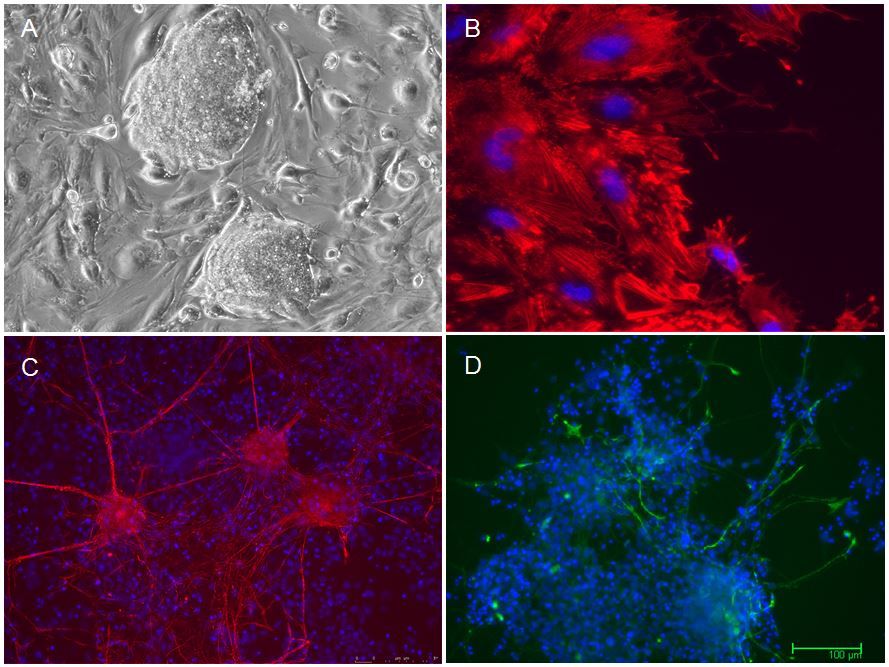
Figure: A. Colony of induced pluripotent stem cells. B.-D.: in vitro differentiation of pluripotent cells towards smooth muscle (B), neurons (C) and glial cells (D).
Heterotopic Ossifications
Heterotopic ossifications (HO) frequently cause complications following orthopedic and trauma surgery and may drastically reduce the postoperative outcome due to pain and joint contracture. Current therapeutic options include NSAID’s and local radiation. However, both options of prevention show disadvantages such as delayed fracture healing and impaired ossification as well as other side effects. Our goal is to investigate novel approaches in the prevention of heterotopic ossification by pharmacologically interfering with the molecular signaling pathways involved in this process. We developed a murine model where HO formation is induced upon Achilles tendon tenotomy. We test different drugs combinations to identify the best treatment to significantly reduce HO bone formation.
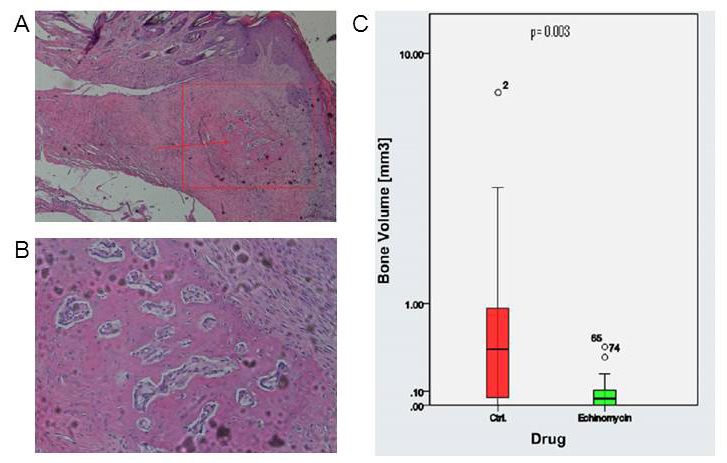
Figure: A. Islet of heterotopic bone within the soft tissue of a specimen’s hind limb; B. Detailed image of islet of heterotopic bone; C. Bone volume in control and Echinomycin group.